A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation
- PMID: 10958694
- PMCID: PMC88774
- DOI: 10.1128/MCB.20.18.6996-7006.2000
A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation
Abstract
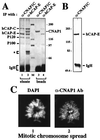
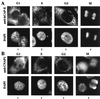
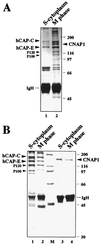
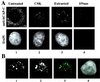
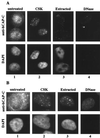
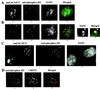
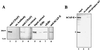
Structural maintenance of chromosomes (SMC) family proteins play critical roles in structural changes of chromosomes. Previously, we identified two human SMC family proteins, hCAP-C and hCAP-E, which form a heterodimeric complex (hCAP-C-hCAP-E) in the cell. Based on the sequence conservation and mitotic chromosome localization, hCAP-C-hCAP-E was determined to be the human ortholog of the Xenopus SMC complex, XCAP-C-XCAP-E. XCAP-C-XCAP-E is a component of the multiprotein complex termed condensin, required for mitotic chromosome condensation in vitro. However, presence of such a complex has not been demonstrated in mammalian cells. Coimmunoprecipitation of the endogenous hCAP-C-hCAP-E complex from HeLa extracts identified a 155-kDa protein interacting with hCAP-C-hCAP-E, termed condensation-related SMC-associated protein 1 (CNAP1). CNAP1 associates with mitotic chromosomes and is homologous to Xenopus condensin component XCAP-D2, indicating the presence of a condensin complex in human cells. Chromosome association of human condensin is mitosis specific, and the majority of condensin dissociates from chromosomes and is sequestered in the cytoplasm throughout interphase. However, a subpopulation of the complex was found to remain on chromosomes as foci in the interphase nucleus. During late G(2)/early prophase, the larger nuclear condensin foci colocalize with phosphorylated histone H3 clusters on partially condensed regions of chromosomes. These results suggest that mitosis-specific function of human condensin may be regulated by cell cycle-specific subcellular localization of the complex, and the nuclear condensin that associates with interphase chromosomes is involved in the reinitiation of mitotic chromosome condensation in conjunction with phosphorylation of histone H3.
Figures

References
-
- Bootsma D, Budke L, Vos O. Studies on synchronous division of tissue culture cells initiated by excess thymidine. Exp Cell Res. 1964;33:301–309. - PubMed
-
- Earnshaw W C, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91:313–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
