Heat shock-induced arrests in different cell cycle phases of rat C6-glioma cells are attenuated in heat shock-primed thermotolerant cells
- PMID: 10959624
- PMCID: PMC6496355
- DOI: 10.1046/j.1365-2184.2000.00175.x
Heat shock-induced arrests in different cell cycle phases of rat C6-glioma cells are attenuated in heat shock-primed thermotolerant cells
Abstract
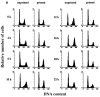
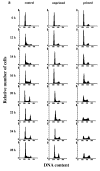
The response kinetics of rat C6 glioma cells to heat shock was investigated by means of flow cytometric DNA measurements and western blot analysis of HSP levels. The results showed that the effects on cell cycle progression are dependent on the cell cycle phase at which heat shock is applied, leading to either G1 or G2/M arrest in randomly proliferating cells. When synchronous cultures were stressed during G0 they were arrested with G1 DNA content and showed prolongation of S and G2 phases after release from the block. In proliferating cells, HSC70 and HSP68 were induced during the recovery and reached maximum levels just before cells were released from the cell cycle blocks. Hyperthermic pretreatment induced thermotolerance both in asynchronous and synchronous cultures as evidenced by the reduced arrest of cell cycle progression after the second heat shock. Thermotolerance development was independent of the cell cycle phase. Pre-treated cells already had high HSP levels and did not further increase the amount of HSP after the second treatment. However, as in unprimed cells, HSP reduction coincided with the release from the cell cycle blocks. These results imply that the cell cycle machinery can be rendered thermotolerant by heat shock pretreatment and supports the assumption that HSP70 family members might be involved in thermotolerance development.
Figures







Similar articles
-
Nuclear translocation of stress protein Hsc70 during S phase in rat C6 glioma cells.Cell Stress Chaperones. 1998 Jun;3(2):94-9. doi: 10.1379/1466-1268(1998)003<0094:ntosph>2.3.co;2. Cell Stress Chaperones. 1998. PMID: 9672244 Free PMC article.
-
Heat-shock gene expression and cell cycle changes during mammalian embryonic development.Dev Genet. 1993;14(2):127-36. doi: 10.1002/dvg.1020140207. Dev Genet. 1993. PMID: 8482017
-
Different constitutive heat shock protein 70 expression during proliferation and differentiation of rat C6 glioma cells.Neurochem Res. 1999 Oct;24(10):1293-9. doi: 10.1023/a:1020933308947. Neurochem Res. 1999. PMID: 10492525
-
Heat shock and the role of the HSPs during neural plate induction in early mammalian CNS and brain development.Cell Mol Life Sci. 1997 Feb;53(2):198-211. doi: 10.1007/pl00000592. Cell Mol Life Sci. 1997. PMID: 9118008 Free PMC article. Review.
-
Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia.Int J Hyperthermia. 1995 Jul-Aug;11(4):459-88. doi: 10.3109/02656739509022483. Int J Hyperthermia. 1995. PMID: 7594802 Review.
Cited by
-
Enhanced expression of 70-kilodalton heat shock protein limits cell division in a sepsis-induced model of acute respiratory distress syndrome.Crit Care Med. 2008 Jan;36(1):246-55. doi: 10.1097/01.CCM.0000295473.56522.EF. Crit Care Med. 2008. PMID: 17989570 Free PMC article.
-
The heat shock-induced cell cycle arrest is attenuated by weak electromagnetic fields.Cell Prolif. 2003 Apr;36(2):101-11. doi: 10.1046/j.1365-2184.2003.00261.x. Cell Prolif. 2003. PMID: 12680877 Free PMC article.
-
Chaperones in cell cycle regulation and mitogenic signal transduction: a review.Cell Prolif. 2000 Dec;33(6):341-65. doi: 10.1046/j.1365-2184.2000.00189.x. Cell Prolif. 2000. PMID: 11101008 Free PMC article. Review.
-
Rapid activation of G2/M checkpoint after hypertonic stress in renal inner medullary epithelial (IME) cells is protective and requires p38 kinase.Proc Natl Acad Sci U S A. 2002 Jan 8;99(1):184-9. doi: 10.1073/pnas.231623498. Epub 2001 Dec 26. Proc Natl Acad Sci U S A. 2002. PMID: 11756692 Free PMC article.
-
Hsp72 expression enhances survival in adenosine triphosphate-depleted renal epithelial cells.Cell Stress Chaperones. 2002 Apr;7(2):137-45. doi: 10.1379/1466-1268(2002)007<0137:heesia>2.0.co;2. Cell Stress Chaperones. 2002. PMID: 12380681 Free PMC article.
References
-
- Beck SC, De Maio A.(1994). Stabilisation of protein synthesis in thermotolerant cells during heat shock. Association of heat shock protein‐72 with ribosomal subunits of polysomes. J. Biol. Chem. 269,21803–21811. - PubMed
-
- Benda P, Lightbody J, Sato G, Levine L, Sweet W.(1968). Differentiated rat glial cell strain in tissue culture. Science 161,370–371. - PubMed
-
- Cellier MFM, Taimi M, Chateau MT, Cannat A, Marti J.(1993). Thermal stress as an inducer of differentiation of U937 cells. Leukemia Res. 17,649–656. - PubMed
-
- Coss RA.(1986). Decay of thermal resistence following acute heating is independent of the G1‐ to S‐phase transition. Rad. Research 107,143–146. - PubMed
-
- De Maio A.(1999). Heat shock proteins: facts, thoughts and dreams. SHOCK 11,1–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

