Geldanamycin, an inhibitor of heat shock protein 90 (Hsp90) mediated signal transduction has anti-inflammatory effects and interacts with glucocorticoid receptor in vivo
- PMID: 10960063
- PMCID: PMC1572305
- DOI: 10.1038/sj.bjp.0703549
Geldanamycin, an inhibitor of heat shock protein 90 (Hsp90) mediated signal transduction has anti-inflammatory effects and interacts with glucocorticoid receptor in vivo
Abstract
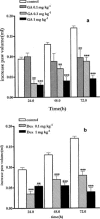
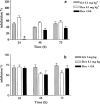
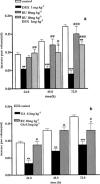
Histamine, vascular endothelial growth factor, acetylcholine, oestrogen as well as fluid shear stress activates a mechanism that recruits heat shock protein 90 to the endothelial nitric oxide synthase. The interaction between Hsp90 and eNOS enhances the activation of the enzyme in cells and in intact blood vessels leading to NO production. Intraplantar administration of carrageenan (50 microl paw(-1)) to mice causes an oedema lasting 72 h. Geldanamycin (0.1, 0.3, 1 mg kg(-1)), a specific inhibitor of Hsp-90, that inhibits endothelium-dependent relaxations of the rat aorta, mesentery and middle artery inhibits carrageenan-induced mouse paw oedema in a dose dependent manner. Co-administration to mice of dexamethasone (1 mg kg(-1)) with geldanamycin (0.3 mg kg(-1)) at anti-inflammatory dose causes a loss of the total anti-inflammatory effect of each agent alone. RU 486 (10 mg kg(-1)), a well known glucocorticoid receptorial antagonist, does not inhibit oedema formation but prevents the anti-inflammatory action of dexamethasone (1 mg kg(-1)). Similarly, RU 486 prevents the anti-inflammatory action of geldanamycin (0.3 mg kg(-1)). In conclusion we have described for the first time that geldanamycin, an inhibitor of Hsp90 dependent signal transduction, is anti-inflammatory in vivo implying that Hsp90 is critical for pathways involved in carrageenan-induced paw oedema. In addition the ability of GA to block NO release and reduce oedema formation suggests a therapeutic rationale for specific inhibitors of Hsp90 as potential anti-inflammatory drugs.
Figures
References
-
- BRESNICK E.H., DALMAN F.C., SANCHEZ E.R., PRATT W.B. Evidence that the 90 kDa heat shock protein is necessary for the steroid binding conformation of the L cell glucocorticoid receptor. J. Biol. Chem. 1989;264:4992–4997. - PubMed
-
- CALHOUN W., CHANG J., CARLSON R.P. Effect of selected antiinflammatory agents and other drugs on zymosan, arachidonic acid, PAF and carrageenan induced paw edema in the mouse. Agent Actions. 1987;21:306–309. - PubMed
-
- DALMAN F.C., BRESNICK E.H., PATEL P.D., PERDEW G.H., WATSON S.J.J., PRATT W.B. Direct evidence that the glucocorticoid receptor binds to Hsp90 at or near the termination of receptor translation in vitro. J. Biol. Chem. 1989;264:19815–19821. - PubMed
-
- GARCIA-CARDEÑA G., FAN R., SHAH V., SORRENTINO R., CIRINO G., PAPAPETROPOULOS A., SESSA W.C. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. - PubMed
-
- GRONEMEYER H., LAUDET V. Transcription factors 3: nuclear receptors. Protein Profile. 1995;2:1173–1308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

