The organic cation transporter OCT2 mediates the uptake of beta-adrenoceptor antagonists across the apical membrane of renal LLC-PK(1) cell monolayers
- PMID: 10960071
- PMCID: PMC1572285
- DOI: 10.1038/sj.bjp.0703518
The organic cation transporter OCT2 mediates the uptake of beta-adrenoceptor antagonists across the apical membrane of renal LLC-PK(1) cell monolayers
Abstract
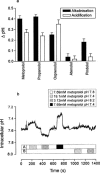
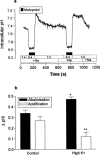
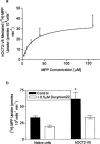
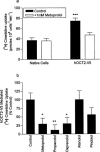
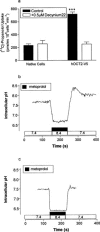
Previous studies have shown that beta-adrenoceptor antagonists may be substrates of organic cation transporters in kidney and lung. In this study we examined the transport of the beta-adrenoreceptor antagonists propranolol and metoprolol, in renal LLC-PK(1) cell monolayers. Experiments with BCECF (2', 7'-bis(2carboxyethyl)-5(6)-carboxyfluorescein) loaded LLC-PK(1) cell monolayers demonstrated that metoprolol and propranolol flux across the basolateral membrane was consistent with non-ionic diffusion. Flux across the apical membrane consisted of both non-ionic diffusion and the uptake of the cationic form of the beta-adrenoceptor antagonists. Uptake of the cationic form of metoprolol across the apical membrane was Na(+)-independent, electrogenic and sensitive to external pH. Furthermore, uptake was sensitive to inhibition by Decynium-22 and the organic cations TEA (tetraethylammonium) and MPP(+) (1-methyl 4-phenylpyridinium). These results, allied with the apical location of the uptake mechanism suggest that beta-adrenoceptor antagonists may be substrates for the organic cation transporter, OCT2. To confirm beta-adrenoceptor antagonists as substrates for OCT2, we demonstrate, in cells transiently transfected with an epitope tagged version of hOCT2 (hOCT2-V5):(1) Decynium-22 sensitive [(14)C]-propranolol uptake, (2) cis-inhibition of OCT2 by a range of beta-adrenoceptor antagonists and (3) metoprolol induced intracellular acidification.
Figures



References
-
- BLEASBY K., CLARK M.A., BROWN C.D.A. Functional expression of an epitope tagged version of the human organic cation transporter hOCT2 in mIMCD3 cells. J. Physiol. 1999;520P:S41.
-
- BLEASBY K., LAUFFART B., CLARK M.A., BROWN C.D.A. DIDS sensitive uptake of cimetidine mediated by an epitope tagged version of the human organic cation transporter hOCT2. FASEB J. 2000;14:A353.
-
- DELLINGER M., PRESSMAN B.C., CALDERON-HIGGINSON C., SAVARAJ N., TAPIERO H., KOLONIAS D., LAMPIDIS T.J. Structural requirements of simple organic cations for recognition by multidrug-resistant cells. Cancer Res. 1992;52:6385–6389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

