The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway
- PMID: 10960075
- PMCID: PMC1572293
- DOI: 10.1038/sj.bjp.0703534
The p38 MAP kinase inhibitor SB203580 enhances nuclear factor-kappa B transcriptional activity by a non-specific effect upon the ERK pathway
Abstract
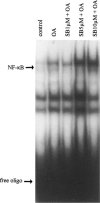
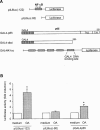
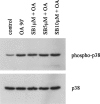
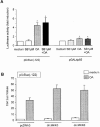
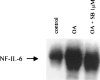
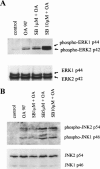
In the present study we investigated a possible role for the p38 mitogen-activated protein (MAP) kinase pathway in mediating nuclear factor-kappa B (NF-kappaB) transcriptional activity in the erythroleukaemic cell line TF-1. TF-1 cells stimulated with the phosphatase inhibitor okadaic acid (OA) demonstrated enhanced NF-kappaB and GAL4p65-regulated transcriptional activity which was associated with elevated p38 phosphorylation. However, pretreatment with the p38 MAPK specific inhibitor SB203580 (1 microM) or overexpression of kinase-deficient mutants of MKK3 or MKK6 did not affect OA-enhanced NF-kappaB transcriptional potency, as determined in transient transfection assays. In fact, 5 and 10 microM SB203580 enhanced rather than inhibited NF-kappaB-mediated promoter activity by 2 fold, which was independent of phosphorylation of the p65 subunit. The SB203580-mediated increase in NF-kappaB transcriptional activity was associated with enhanced phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK), but not p38 kinase. Overexpression of kinase-deficient mutants belonging to the ERK1/2, JNK, and p38 pathways showed that only dominant-negative Raf-1 abrogated SB203580-enhanced NF-kappaB activity. This would implicate the involvement of the ERK1/2 pathway in the enhancing effects of SB203580 on NF-kappaB-mediated gene transcription. This study demonstrates that the p38 MAP kinase pathway is not involved in the OA-induced activation of NF-kappaB. SB203580 at higher concentrations activates the ERK pathway, which subsequently enhances NF-kappaB transcriptional activity.
Figures

Similar articles
-
Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-kappa B-dependent IL-6 expression in human monocytes.J Immunol. 1999 Apr 15;162(8):4893-902. J Immunol. 1999. PMID: 10202034
-
Intracellular signaling in rat cultured vascular smooth muscle cells: roles of nuclear factor-kappaB and p38 mitogen-activated protein kinase on tumor necrosis factor-alpha production.Endocrinology. 1999 Aug;140(8):3562-72. doi: 10.1210/endo.140.8.6914. Endocrinology. 1999. PMID: 10433212
-
NF-κB, ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus.Brain Res Bull. 2013 Oct;99:132-9. doi: 10.1016/j.brainresbull.2013.10.008. Epub 2013 Oct 23. Brain Res Bull. 2013. PMID: 24161765
-
Rac mediates TNF-induced cytokine production via modulation of NF-kappaB.Mol Immunol. 2008 May;45(9):2446-54. doi: 10.1016/j.molimm.2007.12.011. Epub 2008 Feb 6. Mol Immunol. 2008. PMID: 18258304 Review.
-
MAP kinase pathways activated by stress: the p38 MAPK pathway.Crit Care Med. 2000 Apr;28(4 Suppl):N67-77. doi: 10.1097/00003246-200004001-00008. Crit Care Med. 2000. PMID: 10807318 Review.
Cited by
-
Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation.Hum Reprod Update. 2019 May 1;25(3):326-343. doi: 10.1093/humupd/dmy046. Hum Reprod Update. 2019. PMID: 30649364 Free PMC article. Review.
-
Stress-activated kinases as therapeutic targets in pancreatic cancer.World J Gastroenterol. 2021 Aug 14;27(30):4963-4984. doi: 10.3748/wjg.v27.i30.4963. World J Gastroenterol. 2021. PMID: 34497429 Free PMC article. Review.
-
Down-Regulation of Claudin-2 Expression by Cyanidin-3-Glucoside Enhances Sensitivity to Anticancer Drugs in the Spheroid of Human Lung Adenocarcinoma A549 Cells.Int J Mol Sci. 2021 Jan 6;22(2):499. doi: 10.3390/ijms22020499. Int J Mol Sci. 2021. PMID: 33419064 Free PMC article.
-
A novel small molecule inhibitor of p38⍺ MAP kinase augments cardiomyocyte cell cycle entry in response to direct cell cycle stimulation.Br J Pharmacol. 2023 Dec;180(24):3271-3289. doi: 10.1111/bph.16209. Epub 2023 Aug 30. Br J Pharmacol. 2023. PMID: 37547998 Free PMC article.
-
The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling.J Virol. 2016 Oct 14;90(21):9743-9757. doi: 10.1128/JVI.01382-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535052 Free PMC article.
References
-
- AKIRA S., KISHIMOTO T. NF-IL6 and NF-kappa B in cytokine gene regulation. Adv. Immunol. 1997;65:1–46. - PubMed
-
- ALPERT D., SCHWENGER P., HAN J., VILCEK J. Cell stress and MKK6b-mediated p38 MAP kinase activation inhibit Tumor Necrosis Factor-induced IκB phosphorylation and NF-κB activation. J. Biol. Chem. 1999;274:22176–22183. - PubMed
-
- BEG A.A., BALTIMORE D. An essential role for NF-kappa B in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. - PubMed
-
- BEG A.A., SHA W.C., BRONSON R.T., GHOSH S., BALTIMORE D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. - PubMed
-
- BERGMANN M., HART L., LINDSAY M., BARNES P.J., NEWTON R. IkappaBalpha degradation and nuclear factor-kappaB DNA binding are insufficient for interleukin-1 beta and tumor necrosis factor-alpha-induced kappaB-dependent transcription. J. Biol. Chem. 1998;273:6607–6610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

