Mechanisms of the thapsigargin-induced Ca(2+) entry in in situ endothelial cells of the porcine aortic valve and the endothelium-dependent relaxation in the porcine coronary artery
- PMID: 10960077
- PMCID: PMC1572304
- DOI: 10.1038/sj.bjp.0703548
Mechanisms of the thapsigargin-induced Ca(2+) entry in in situ endothelial cells of the porcine aortic valve and the endothelium-dependent relaxation in the porcine coronary artery
Abstract
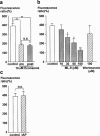
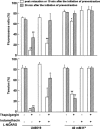
The mechanisms of the thapsigargin (TG)-induced capacitative Ca(2+) entry in in situ endothelial cells and its role in the regulation of arterial tone were investigated using front-surface fluorimetry and fura-2-loaded strips of porcine aortic valve and coronary artery. In the presence of extracellular Ca(2+), TG induced an initial rapid and a subsequent sustained elevation of cytosolic Ca(2+) concentration ([Ca(2+)](i)) in valvular strips. In the absence of extracellular Ca(2+), TG induced only a transient increase in [Ca(2+)](i). The TG-induced sustained elevation of [Ca(2+)](i) in endothelial cells was inhibited completely by 1 mM Ni(2+) and partly by 10 microM econazole and 30 microM ML-9, but not by 900 ng ml(-1) pertussis toxin or 100 microM wortmannin. Therefore, cytochrome P450 and protein phosphorylation are suggested to be involved in the TG-induced Ca(2+) influx in in situ endothelial cells. TG induced an endothelium-dependent large relaxation consisting of an initial and a late sustained relaxation in coronary arterial strip precontracted with U46619 (a thromboxane A2 analogue). Indomethacin alone had no effect, while indomethacin plus N(omega)-nitro-L-arginine (L-NOARG) markedly inhibited the sustained phase and slightly inhibited the initial phase of the TG-induced relaxation. TG induced a smaller but sustained relaxation during the 40 mM K(+)-induced precontraction than that seen during the U46619-induced precontraction. This relaxation was completely abolished by the pretreatment with indomethacin plus L-NOARG. In conclusion, both nitric oxide (NO) and endothelium-derived hyperpolarizing factor were suggested to mediate the TG-induced relaxation, while NO plays a major role in the sustained relaxation. The TG-induced sustained [Ca(2+)](i) elevation in endothelial cells was thus suggested to be mainly linked to the sustained production of NO.
Figures



References
-
- ALVAREZ J., MONTERO M., GARCIA-SANCHO J. Cytochrome P450 may regulate plasma membrane Ca2+ permeability according to the filling state of the intracellular Ca2+ stores. FASEB J. 1992;6:786–792. - PubMed
-
- AOKI H., KOBAYASHI S., NISHIMURA J., YAMAMOTO H., KANAIDE H. Endothelin induces the Ca2+-transient in endothelial cells in situ. Biochem. Biophys. Res. Commun. 1991;181:1352–1357. - PubMed
-
- BIRD G.S., PUTNEY J.W.J. Inhibition of thapsigargin-induced calcium entry by microinjected guanine nucleotide analogues. Evidence for the involvement of a small G-protein in capacitative calcium entry. J. Biol. Chem. 1993;268:21486–21488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

