The flagellar filament of Rhodobacter sphaeroides: pH-induced polymorphic transitions and analysis of the fliC gene
- PMID: 10960108
- PMCID: PMC94672
- DOI: 10.1128/JB.182.18.5218-5224.2000
The flagellar filament of Rhodobacter sphaeroides: pH-induced polymorphic transitions and analysis of the fliC gene
Abstract
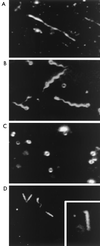
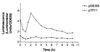
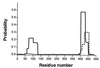
Flagellar motility in Rhodobacter sphaeroides is notably different from that in other bacteria. R. sphaeroides moves in a series of runs and stops produced by the intermittent rotation of the flagellar motor. R. sphaeroides has a single, plain filament whose conformation changes according to flagellar motor activity. Conformations adopted during swimming include coiled, helical, and apparently straight forms. This range of morphological transitions is larger than that in other bacteria, where filaments alternate between left- and right-handed helical forms. The polymorphic ability of isolated R. sphaeroides filaments was tested in vitro by varying pH and ionic strength. The isolated filaments could form open-coiled, straight, normal, or curly conformations. The range of transitions made by the R. sphaeroides filament differs from that reported for Salmonella enterica serovar Typhimurium. The sequence of the R. sphaeroides fliC gene, which encodes the flagellin protein, was determined. The gene appears to be controlled by a sigma(28)-dependent promoter. It encodes a predicted peptide of 493 amino acids. Serovar Typhimurium mutants with altered polymorphic ability usually have amino acid changes at the terminal portions of flagellin or a deletion in the central region. There are no obvious major differences in the central regions to explain the difference in polymorphic ability. In serovar Typhimurium filaments, the termini of flagellin monomers have a coiled-coil conformation. The termini of R. sphaeroides flagellin are predicted to have a lower probability of coiled coils than are those of serovar Typhimurium flagellin. This may be one reason for the differences in polymorphic ability between the two filaments.
Figures

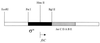


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

