A point mutation in the pore region alters gating, Ca(2+) blockage, and permeation of olfactory cyclic nucleotide-gated channels
- PMID: 10962010
- PMCID: PMC2233693
- DOI: 10.1085/jgp.116.3.311
A point mutation in the pore region alters gating, Ca(2+) blockage, and permeation of olfactory cyclic nucleotide-gated channels
Abstract
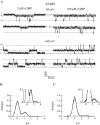
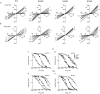
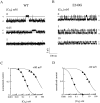
Upon stimulation by odorants, Ca(2+) and Na(+) enter the cilia of olfactory sensory neurons through channels directly gated by cAMP. Cyclic nucleotide-gated channels have been found in a variety of cells and extensively investigated in the past few years. Glutamate residues at position 363 of the alpha subunit of the bovine retinal rod channel have previously been shown to constitute a cation-binding site important for blockage by external divalent cations and to control single-channel properties. It has therefore been assumed, but not proven, that glutamate residues at the corresponding position of the other cyclic nucleotide-gated channels play a similar role. We studied the corresponding glutamate (E340) of the alpha subunit of the bovine olfactory channel to determine its role in channel gating and in permeation and blockage by Ca(2+) and Mg(2+). E340 was mutated into either an aspartate, glycine, glutamine, or asparagine residue and properties of mutant channels expressed in Xenopus laevis oocytes were measured in excised patches. By single-channel recordings, we demonstrated that the open probabilities in the presence of cGMP or cAMP were decreased by the mutations, with a larger decrease observed on gating by cAMP. Moreover, we observed that the mutant E340N presented two conductance levels. We found that both external Ca(2+) and Mg(2+) powerfully blocked the current in wild-type and E340D mutants, whereas their blockage efficacy was drastically reduced when the glutamate charge was neutralized. The inward current carried by external Ca(2+) relative to Na(+) was larger in the E340G mutant compared with wild-type channels. In conclusion, we have confirmed that the residue at position E340 of the bovine olfactory CNG channel is in the pore region, controls permeation and blockage by external Ca(2+) and Mg(2+), and affects channel gating by cAMP more than by cGMP.
Figures
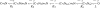
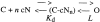



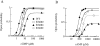

Similar articles
-
Co-expression of wild-type and mutant olfactory cyclic nucleotide-gated channels: restoration of the native sensitivity to Ca(2+) and Mg(2+) blockage.Neuroreport. 2001 Aug 8;12(11):2363-7. doi: 10.1097/00001756-200108080-00016. Neuroreport. 2001. PMID: 11496111
-
Mechanisms of modulation by internal protons of cyclic nucleotide-gated channels cloned from sensory receptor cells.Proc Biol Sci. 1997 Aug 22;264(1385):1157-65. doi: 10.1098/rspb.1997.0160. Proc Biol Sci. 1997. PMID: 9308192 Free PMC article.
-
The interaction of Na(+) and K(+) in the pore of cyclic nucleotide-gated channels.Biophys J. 2000 Nov;79(5):2475-93. doi: 10.1016/S0006-3495(00)76490-3. Biophys J. 2000. PMID: 11053124 Free PMC article.
-
Cyclic nucleotide-gated channels in visual and olfactory transduction.Biophys Chem. 1995 Aug;55(3):185-96. doi: 10.1016/0301-4622(94)00153-b. Biophys Chem. 1995. PMID: 7542935 Review.
-
Cyclic nucleotide-gated ion channels.Physiol Rev. 2002 Jul;82(3):769-824. doi: 10.1152/physrev.00008.2002. Physiol Rev. 2002. PMID: 12087135 Review.
Cited by
-
Mutation of the pore glutamate affects both cytoplasmic and external dequalinium block in the rat olfactory CNGA2 channel.Eur Biophys J. 2005 Jul;34(5):442-53. doi: 10.1007/s00249-005-0479-7. Epub 2005 Jun 1. Eur Biophys J. 2005. PMID: 15928936
-
Molecular basis of an inherited form of incomplete achromatopsia.J Neurosci. 2004 Jan 7;24(1):138-47. doi: 10.1523/JNEUROSCI.3883-03.2004. J Neurosci. 2004. PMID: 14715947 Free PMC article.
-
Mediation of mammalian olfactory response by presence of odor-evoked potassium current.Front Allergy. 2024 Oct 16;5:1478529. doi: 10.3389/falgy.2024.1478529. eCollection 2024. Front Allergy. 2024. PMID: 39479387 Free PMC article.
-
Structural mechanisms of gating and selectivity of human rod CNGA1 channel.Neuron. 2021 Apr 21;109(8):1302-1313.e4. doi: 10.1016/j.neuron.2021.02.007. Epub 2021 Mar 1. Neuron. 2021. PMID: 33651975 Free PMC article.
-
Inactivation of TRPM2 channels by extracellular divalent copper.PLoS One. 2014 Nov 11;9(11):e112071. doi: 10.1371/journal.pone.0112071. eCollection 2014. PLoS One. 2014. PMID: 25386648 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

