The EEEE locus is the sole high-affinity Ca(2+) binding structure in the pore of a voltage-gated Ca(2+) channel: block by ca(2+) entering from the intracellular pore entrance
- PMID: 10962013
- PMCID: PMC2233694
- DOI: 10.1085/jgp.116.3.349
The EEEE locus is the sole high-affinity Ca(2+) binding structure in the pore of a voltage-gated Ca(2+) channel: block by ca(2+) entering from the intracellular pore entrance
Abstract
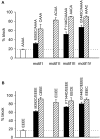
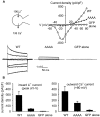
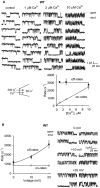
Selective permeability in voltage-gated Ca(2+) channels is dependent upon a quartet of pore-localized glutamate residues (EEEE locus). The EEEE locus is widely believed to comprise the sole high-affinity Ca(2+) binding site in the pore, which represents an overturning of earlier models that had postulated two high-affinity Ca(2+) binding sites. The current view is based on site-directed mutagenesis work in which Ca(2+) binding affinity was attenuated by single and double substitutions in the EEEE locus, and eliminated by quadruple alanine (AAAA), glutamine (QQQQ), or aspartate (DDDD) substitutions. However, interpretation of the mutagenesis work can be criticized on the grounds that EEEE locus mutations may have additionally disrupted the integrity of a second, non-EEEE locus high-affinity site, and that such a second site may have remained undetected because the mutated pore was probed only from the extracellular pore entrance. Here, we describe the results of experiments designed to test the strength of these criticisms of the single high-affinity locus model of selective permeability in Ca(2+) channels. First, substituted-cysteine accessibility experiments indicate that pore structure in the vicinity of the EEEE locus is not extensively disrupted as a consequence of the quadruple AAAA mutations, suggesting in turn that the quadruple mutations do not distort pore structure to such an extent that a second high affinity site would likely be destroyed. Second, the postulated second high-affinity site was not detected by probing from the intracellularly oriented pore entrance of AAAA and QQQQ mutants. Using inside-out patches, we found that, whereas micromolar Ca(2+) produced substantial block of outward Li(+) current in wild-type channels, internal Ca(2+) concentrations up to 1 mM did not produce detectable block of outward Li(+) current in the AAAA or QQQQ mutants. These results indicate that the EEEE locus is indeed the sole high-affinity Ca(2+) binding locus in the pore of voltage-gated Ca(2+) channels.
Figures



Similar articles
-
Ion interactions in the high-affinity binding locus of a voltage-gated Ca(2+) channel.J Gen Physiol. 2000 Oct;116(4):569-86. doi: 10.1085/jgp.116.4.569. J Gen Physiol. 2000. PMID: 11004206 Free PMC article.
-
Side chain orientation in the selectivity filter of a voltage-gated Ca2+ channel.J Biol Chem. 2000 Oct 13;275(41):31778-85. doi: 10.1074/jbc.M004829200. J Biol Chem. 2000. PMID: 10934200
-
Molecular determinant for specific Ca/Ba selectivity profiles of low and high threshold Ca2+ channels.J Gen Physiol. 2007 Oct;130(4):415-25. doi: 10.1085/jgp.200709771. J Gen Physiol. 2007. PMID: 17893194 Free PMC article.
-
Molecular pore structure of voltage-gated sodium and calcium channels.Braz J Med Biol Res. 1994 Dec;27(12):2781-802. Braz J Med Biol Res. 1994. PMID: 7550000 Review.
-
Permeation and selectivity in calcium channels.Annu Rev Physiol. 2003;65:133-59. doi: 10.1146/annurev.physiol.65.092101.142345. Epub 2002 Nov 21. Annu Rev Physiol. 2003. PMID: 12471162 Review.
Cited by
-
Deciphering Ca2+ permeation and valence selectivity in CaV1: Molecular dynamics simulations reveal the three-ion knock-on mechanism.Proc Natl Acad Sci U S A. 2025 Jun 3;122(22):e2424694122. doi: 10.1073/pnas.2424694122. Epub 2025 May 29. Proc Natl Acad Sci U S A. 2025. PMID: 40440072 Free PMC article.
-
How "Pharmacoresistant" is Cav2.3, the Major Component of Voltage-Gated R-type Ca2+ Channels?Pharmaceuticals (Basel). 2013 May 27;6(6):759-76. doi: 10.3390/ph6060759. Pharmaceuticals (Basel). 2013. PMID: 24276260 Free PMC article.
-
Calcium Ion Channels in Saccharomyces cerevisiae.J Fungi (Basel). 2023 Apr 28;9(5):524. doi: 10.3390/jof9050524. J Fungi (Basel). 2023. PMID: 37233235 Free PMC article. Review.
-
Identification and analysis of cation channel homologues in human pathogenic fungi.PLoS One. 2012;7(8):e42404. doi: 10.1371/journal.pone.0042404. Epub 2012 Aug 2. PLoS One. 2012. PMID: 22876320 Free PMC article.
-
Molecular determinants and role of an anion binding site in the external mouth of the CFTR chloride channel pore.J Physiol. 2003 Jun 1;549(Pt 2):387-97. doi: 10.1113/jphysiol.2002.038232. Epub 2003 Apr 4. J Physiol. 2003. PMID: 12679372 Free PMC article.
References
-
- Akabas M.H., Stauffer D.A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. - PubMed
-
- Armstrong C.M., Neyton J. Ion permeation through calcium channelsa one-site model. Ann. NY Acad. Sci. 1991;635:18–25. - PubMed
-
- Barik S. Site-directed mutagenesis by PCRsubstitution, insertion, deletion, and gene fusion. In: Sarkar G., editor. Methods in Neuroscience. Vol. 26. Academic Press; San Diego, CA: 1995. pp. 309–323.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

