The molecular basis for Na-dependent phosphate transport in human erythrocytes and K562 cells
- PMID: 10962014
- PMCID: PMC2233690
- DOI: 10.1085/jgp.116.3.363
The molecular basis for Na-dependent phosphate transport in human erythrocytes and K562 cells
Abstract
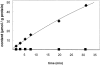
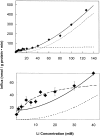
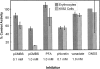
The kinetics of sodium-stimulated phosphate flux and phosphate-stimulated sodium flux in human red cells have been previously described (Shoemaker, D.G., C.A. Bender, and R.B. Gunn. 1988. J. Gen. Physiol. 92:449-474). However, despite the identification of multiple isoforms in three gene families (Timmer, R.T., and R.B. Gunn. 1998. Am. J. Physiol. Cell Physiol. 274:C757-C769), the molecular basis for the sodium-phosphate cotransporter in erythrocytes is unknown. Most cells express multiple isoforms, thus disallowing explication of isoform-specific kinetics and function. We have found that erythrocyte membranes express one dominant isoform, hBNP-1, to which the kinetics can thus be ascribed. In addition, because the erythrocyte Na-PO(4) cotransporter can also mediate Li-PO(4) cotransport, it has been suggested that this transporter functions as the erythrocyte Na-Li exchanger whose activity is systematically altered in patients with bipolar disease and patients with essential hypertension. To determine the molecular basis for the sodium-phosphate cotransporter, we reasoned that if the kinetics of phosphate transport in a nucleated erythroid-like cell paralleled those of the Na-activated pathway in anucleated erythrocytes and yet were distinct from those known for other Na-PO(4) cotransporters, then the expressed genes may be the same in both cell types. In this study, we show that the kinetics of sodium phosphate cotransport were similar in anuclear human erythrocytes and K562 cells, a human erythroleukemic cell line. Although the erythrocyte fluxes were 750-fold smaller, the half-activation concentrations for phosphate and sodium and the relative cation specificities for activation of (32)PO(4) influx were similar. Na-activation curves for both cell types showed cooperativity consistent with the reported stoichiometry of more than one Na cotransported per PO(4). In K562 cells, external lithium activation of phosphate influx was also cooperative. Inhibition by arsenate, K(I) = 2.6-2.7 mM, and relative inhibition by amiloride, amiloride analogs, phosphonoformate, and phloretin were similar. These characteristics were different from those reported for hNaPi-3 and hPiT-1 in other systems. PCR analysis of sodium-phosphate cotransporter isoforms in K562 cells demonstrated the presence of mRNAs for hPiT-1, hPiT-2, and hBNP-1. The mRNAs for hNaPi-10 and hNaPi-3, the other two known isoforms, were absent. Western analysis of erythrocytes and K562 cells with isoform-specific antibodies detected the presence of only hBNP-1, an isoform expressed in brain neurons and glia. The similarities in the kinetics and the expression of only hBNP-1 protein in the two cell types is strong evidence that hBNP-1 is the erythrocyte and K562 cell sodium-phosphate cotransporter.
Figures







Similar articles
-
Sodium-phosphate cotransport in human red blood cells. Kinetics and role in membrane metabolism.J Gen Physiol. 1988 Oct;92(4):449-74. doi: 10.1085/jgp.92.4.449. J Gen Physiol. 1988. PMID: 3204363 Free PMC article.
-
Kinetic evidence that the Na-PO4 cotransporter is the molecular mechanism for Na/Li exchange in human red blood cells.Am J Physiol Cell Physiol. 2003 Aug;285(2):C446-56. doi: 10.1152/ajpcell.00606.2002. Epub 2003 Apr 2. Am J Physiol Cell Physiol. 2003. PMID: 12672655
-
Phosphate transport by the human renal cotransporter NaPi-3 expressed in HEK-293 cells.Am J Physiol. 1998 Mar;274(3):C757-69. doi: 10.1152/ajpcell.1998.274.3.C757. Am J Physiol. 1998. PMID: 9530108
-
The renal type II Na+/phosphate cotransporter.J Bioenerg Biomembr. 1998 Apr;30(2):187-94. doi: 10.1023/a:1020525409894. J Bioenerg Biomembr. 1998. PMID: 9672240 Review.
-
Proximal tubular phosphate reabsorption: molecular mechanisms.Physiol Rev. 2000 Oct;80(4):1373-409. doi: 10.1152/physrev.2000.80.4.1373. Physiol Rev. 2000. PMID: 11015617 Review.
Cited by
-
A genome-wide association study of red blood cell traits using the electronic medical record.PLoS One. 2010 Sep 28;5(9):e13011. doi: 10.1371/journal.pone.0013011. PLoS One. 2010. PMID: 20927387 Free PMC article.
-
Unidirectional Flux Balance of Monovalent Ions in Cells with Na/Na and Li/Na Exchange: Experimental and Computational Studies on Lymphoid U937 Cells.PLoS One. 2016 May 9;11(5):e0153284. doi: 10.1371/journal.pone.0153284. eCollection 2016. PLoS One. 2016. PMID: 27159324 Free PMC article.
-
Caveola-dependent endocytic entry of amphotropic murine leukemia virus.J Virol. 2005 Aug;79(16):10776-87. doi: 10.1128/JVI.79.16.10776-10787.2005. J Virol. 2005. PMID: 16051869 Free PMC article.
-
Tetrodotoxin-sensitive Na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts.Proc Natl Acad Sci U S A. 2004 Aug 17;101(33):12370-4. doi: 10.1073/pnas.0404228101. Epub 2004 Aug 3. Proc Natl Acad Sci U S A. 2004. PMID: 15292511 Free PMC article.
-
Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1.Am J Physiol Cell Physiol. 2021 Dec 1;321(6):C1028-C1059. doi: 10.1152/ajpcell.00275.2021. Epub 2021 Oct 20. Am J Physiol Cell Physiol. 2021. PMID: 34669510 Free PMC article. Review.
References
-
- Adragna N.C., Canessa M.L., Solomon H., Slater E., Tosteson D.C. Red cell lithium-sodium countertransport and sodium–potassium cotransport in patients with essential hypertension. Hypertension (Dallas). 1982;4:795–804. - PubMed
-
- Azzarolo A.M., Ritchie G., Quamme G. Inhibition of sodium-phosphate cotransport in renal brush-border membranes with the stilbenedisulfonate, H2-DIDS. Biochim. Biophys. Acta. 1991;1069:70–76. - PubMed
-
- Canessa M., Adragna N., Solomon H.S., Connolly T.M., Tosteson D.C. Increased sodium–lithium countertransport in red cells of patients with essential hypertension. N. Engl. J. Med. 1980;302:772–776. - PubMed

