MinK subdomains that mediate modulation of and association with KvLQT1
- PMID: 10962015
- PMCID: PMC2233688
- DOI: 10.1085/jgp.116.3.379
MinK subdomains that mediate modulation of and association with KvLQT1
Abstract
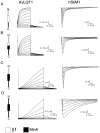
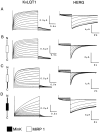
KvLQT1 is a voltage-gated potassium channel expressed in cardiac cells that is critical for myocardial repolarization. When expressed alone in heterologous expression systems, KvLQT1 channels exhibit a rapidly activating potassium current that slowly deactivates. MinK, a 129 amino acid protein containing one transmembrane-spanning domain modulates KvLQT1, greatly slowing activation, increasing current amplitude, and removing inactivation. Using deletion and chimeric analysis, we have examined the structural determinants of MinK effects on gating modulation and subunit association. Coexpression of KvLQT1 with a MinK COOH-terminus deletion mutant (MinK DeltaCterm) in Xenopus oocytes resulted in a rapidly activated potassium current closely resembling currents recorded from oocytes expressing KvLQT1 alone, indicating that this region is necessary for modulation. To determine whether MinK DeltaCterm was associated with KvLQT1, a functional tag (G55C) that confers susceptibility to partial block by external cadmium was engineered into the transmembrane domain of MinK DeltaCterm. Currents derived from coexpression of KvLQT1 with MinK DeltaCterm were cadmium sensitive, suggesting that MinK DeltaCterm does associate with KvLQT1, but does not modulate gating. To determine which MinK regions are sufficient for KvLQT1 association and modulation, chimeras were generated between MinK and the Na(+) channel beta1 subunit. Chimeras between MinK and beta1 could only modulate KvLQT1 if they contained both the MinK transmembrane domain and COOH terminus, suggesting that the MinK COOH terminus alone is not sufficient for KvLQT1 modulation, and requires an additional, possibly associative interaction between the MinK transmembrane domain and KvLQT1. To identify the MinK subdomains necessary for gating modulation, deletion mutants were designed and coexpressed with KvLQT1. A MinK construct with amino acid residues 94-129 deleted retained the ability to modulate KvLQT1 gating, identifying the COOH-terminal region critical for gating modulation. Finally, MinK/MiRP1 (MinK related protein-1) chimeras were generated to investigate the difference between these two closely related subunits in their ability to modulate KvLQT1. The results from this analysis indicate that MiRP1 cannot modulate KvLQT1 due to differences within the transmembrane domain. Our results allow us to identify the MinK subdomains that mediate KvLQT1 association and modulation.
Figures



Similar articles
-
MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel.J Biol Chem. 1998 Dec 18;273(51):34069-74. doi: 10.1074/jbc.273.51.34069. J Biol Chem. 1998. PMID: 9852064
-
Long QT syndrome-associated mutations in the S4-S5 linker of KvLQT1 potassium channels modify gating and interaction with minK subunits.J Biol Chem. 1999 Jul 23;274(30):21063-70. doi: 10.1074/jbc.274.30.21063. J Biol Chem. 1999. PMID: 10409658
-
Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome.J Gen Physiol. 1998 Dec;112(6):651-63. doi: 10.1085/jgp.112.6.651. J Gen Physiol. 1998. PMID: 9834138 Free PMC article.
-
Properties and regulation of the minK potassium channel protein.Physiol Rev. 1997 Jul;77(3):627-41. doi: 10.1152/physrev.1997.77.3.627. Physiol Rev. 1997. PMID: 9234960 Review.
-
KCNE regulation of KvLQT1 channels: structure-function correlates.Trends Cardiovasc Med. 2002 May;12(4):182-7. doi: 10.1016/s1050-1738(02)00158-5. Trends Cardiovasc Med. 2002. PMID: 12069759 Review.
Cited by
-
Intracellular trafficking of the KV1.3 potassium channel is regulated by the prodomain of a matrix metalloprotease.J Biol Chem. 2013 Mar 1;288(9):6451-64. doi: 10.1074/jbc.M112.421495. Epub 2013 Jan 8. J Biol Chem. 2013. PMID: 23300077 Free PMC article.
-
Domain structure and function of matrix metalloprotease 23 (MMP23): role in potassium channel trafficking.Cell Mol Life Sci. 2014 Apr;71(7):1191-210. doi: 10.1007/s00018-013-1431-0. Epub 2013 Aug 3. Cell Mol Life Sci. 2014. PMID: 23912897 Free PMC article. Review.
-
The Polymorphic Analysis of the Human Potassium Channel KCNE Gene Family in Meniere's Disease-A Preliminary Study.J Int Adv Otol. 2019 Apr;15(1):130-134. doi: 10.5152/iao.2019.5076. J Int Adv Otol. 2019. PMID: 31058602 Free PMC article.
-
Probing Structural Dynamics and Topology of the KCNE1 Membrane Protein in Lipid Bilayers via Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy.Biochemistry. 2015 Oct 20;54(41):6402-12. doi: 10.1021/acs.biochem.5b00505. Epub 2015 Oct 7. Biochemistry. 2015. PMID: 26418890 Free PMC article.
-
KCNE variants reveal a critical role of the beta subunit carboxyl terminus in PKA-dependent regulation of the IKs potassium channel.Channels (Austin). 2009 Jan-Feb;3(1):16-24. doi: 10.4161/chan.3.1.7387. Epub 2009 Jan 7. Channels (Austin). 2009. PMID: 19077539 Free PMC article.
References
-
- Abbott G.W., Sesti F., Splawski I., Buck M.E., Lehmann M.H., Timaothy K.W., Keating M.T., Goldstein S.A.N. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Barhanin J., Lesage F., Guillemare E., Fink M., Lazdunski M., Romey G. KvLQT1 and IsK (MinK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Ben-Efraim I., Shai Y., Attali B. Cytoplasmic and extracellular IsK peptides activate endogenous K+ and Cl− in Xenopus oocytesevidence for regulatory function. J. Biol. Chem. 1996;271:8768–8771. - PubMed
-
- Bianchi L., Shen Z., Dennis A.T., Priori S.G., Napolitano C., Ronchetti E., Bryskin R., Shwartz P.J., Brown A.M. Cellular dysfunction of LQT5-MinK mutantsabnormalities of IKs, IKr and trafficking in long QT syndrome. Hum. Mol. Genet. 1999;8:1499–1507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

