Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity
- PMID: 10962017
- PMCID: PMC2233685
- DOI: 10.1085/jgp.116.3.411
Role of the beta1 subunit in large-conductance Ca(2+)-activated K(+) channel gating energetics. Mechanisms of enhanced Ca(2+) sensitivity
Abstract
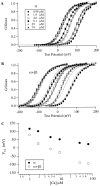
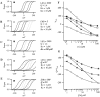
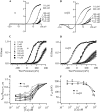
Over the past few years, it has become clear that an important mechanism by which large-conductance Ca(2+)-activated K(+) channel (BK(Ca)) activity is regulated is the tissue-specific expression of auxiliary beta subunits. The first of these to be identified, beta1, is expressed predominately in smooth muscle and causes dramatic effects, increasing the apparent affinity of the channel for Ca(2+) 10-fold at 0 mV, and shifting the range of voltages over which the channel activates -80 mV at 9.1 microM Ca(2+). With this study, we address the question: which aspects of BK(Ca) gating are altered by beta1 to bring about these effects: Ca(2+) binding, voltage sensing, or the intrinsic energetics of channel opening? The approach we have taken is to express the beta1 subunit together with the BK(Ca) alpha subunit in Xenopus oocytes, and then to compare beta1's steady state effects over a wide range of Ca(2+) concentrations and membrane voltages to those predicted by allosteric models whose parameters have been altered to mimic changes in the aspects of gating listed above. The results of our analysis suggest that much of beta1's steady state effects can be accounted for by a reduction in the intrinsic energy the channel must overcome to open and a decrease in its voltage sensitivity, with little change in the affinity of the channel for Ca(2+) when it is either open or closed. Interestingly, however, the small changes in Ca(2+) binding affinity suggested by our analysis (K(c) 7.4 microM --> 9.6 microM; K(o) = 0.80 microM --> 0.65 microM) do appear to be functionally important. We also show that beta1 affects the mSlo conductance-voltage relation in the essential absence of Ca(2+), shifting it +20 mV and reducing its apparent gating charge 38%, and we develop methods for distinguishing between alterations in Ca(2+) binding and other aspects of BK(Ca) channel gating that may be of general use.
Figures

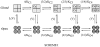






References
-
- Adelman J.P., Shen K.Z., Kavanaugh M.P., Warren R.A., Wu Y.N., Lagrutta A., Bond C.T., North R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. - PubMed
-
- Atkinson N.S., Robertson G.A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
-
- Bers D., Patton C., Nuccitelli R. A practical guide to the preparation of Ca buffers. Methods Cell Biol. 1994;40:3–29. - PubMed
-
- Brenner R., Jegla T.J., Wickenden A., Liu Y., Aldrich R.W. Cloning and functional characterization of novel potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 2000;275:6453–6461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

