Fast and slow gating relaxations in the muscle chloride channel CLC-1
- PMID: 10962018
- PMCID: PMC2233683
- DOI: 10.1085/jgp.116.3.433
Fast and slow gating relaxations in the muscle chloride channel CLC-1
Abstract
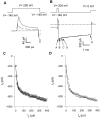
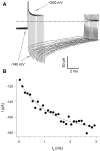
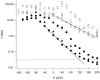
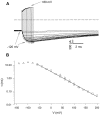
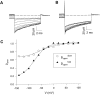
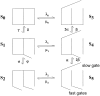
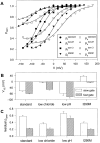
Gating of the muscle chloride channel CLC-1 involves at least two processes evidenced by double-exponential current relaxations when stepping the voltage to negative values. However, there is little information about the gating of CLC-1 at positive voltages. Here, we analyzed macroscopic gating of CLC-1 over a large voltage range (from -160 to +200 mV). Activation was fast at positive voltages but could be easily followed using envelope protocols that employed a tail pulse to -140 mV after stepping the voltage to a certain test potential for increasing durations. Activation was biexponential, demonstrating the presence of two gating processes. Both time constants became exponentially faster at positive voltages. A similar voltage dependence was also seen for the fast gate time constant of CLC-0. The voltage dependence of the time constant of the fast process of CLC-1, tau(f), was steeper than that of the slow one, tau(s) (apparent activation valences were z(f) approximately -0. 79 and z(s) approximately -0.42) such that at +200 mV the two processes became kinetically distinct by almost two orders of magnitude (tau(f) approximately 16 micros, tau(s) approximately 1 ms). This voltage dependence is inconsistent with a previously published gating model for CLC-1 (Fahlke, C., A. Rosenbohm, N. Mitrovic, A.L. George, and R. Rüdel. 1996. Biophys. J. 71:695-706). The kinetic difference at 200 mV allowed us to separate the steady state open probabilities of the two processes assuming that they reflect two parallel (not necessarily independent) gates that have to be open simultaneously to allow ion conduction. Both open probabilities could be described by Boltzmann functions with gating valences around one and with nonzero "offsets" at negative voltages, indicating that the two "gates" never close completely. For comparison with single channel data and to correlate the two gating processes with the two gates of CLC-0, we characterized their voltage, pH(int), and [Cl](ext) dependence, and the dominant myotonia inducing mutation, I290M. Assuming a double-barreled structure of CLC-1, our results are consistent with the identification of the fast and slow gating processes with the single-pore and the common-pore gate, respectively.
Figures


References
-
- Fahlke C., Rhodes T.H., Desai R.R., George A.L., Jr. Pore stoichiometry of a voltage-gated chloride channel. Nature. 1998;394:687–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

