Human renal cell carcinoma expresses distinct binding sites for growth hormone-releasing hormone
- PMID: 10962030
- PMCID: PMC27063
- DOI: 10.1073/pnas.180313097
Human renal cell carcinoma expresses distinct binding sites for growth hormone-releasing hormone
Abstract
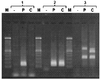
Antagonists of growth hormone-releasing hormone (GHRH) inhibit the proliferation of various human cancers in vitro and in vivo by mechanisms that include apparent direct effects through specific binding sites expressed on tumors and that differ from pituitary human GHRH (hGHRH) receptors. In this study, GHRH antagonist JV-1-38 (20 microgram/day per animal s.c.) inhibited the growth of orthotopic CAKI-1 human renal cell carcinoma (RCC) by 83% and inhibited the development of metastases to lung and lymph nodes. Using ligand competition assays with (125)I-labeled GHRH antagonist JV-1-42, we demonstrated the presence of specific high-affinity (K(d) = 0.25 +/- 0.03 nM) binding sites for GHRH with a maximal binding capacity (B(max)) of 70.2 +/- 4.1 fmol/mg of membrane protein in CAKI-1 tumors. These receptors bind GHRH antagonists preferentially and display a lower affinity for hGHRH. The binding of (125)I-JV-1-42 is not inhibited by vasoactive intestinal peptide (VIP)-related peptides sharing structural homology with hGHRH. The receptors for GHRH antagonists on CAKI-1 tumors are distinct from binding sites detected with (125)I-VIP (K(d) = 0.89 +/- 0.14 nM; B(max) = 183.5 +/- 2.6 fmol/mg of protein) and also have different characteristics from GHRH receptors on rat pituitary as documented by the insignificant binding of [His(1),(125)I-Tyr(10), Nle(27)]hGHRH(1-32)NH(2). Reverse transcription-PCR revealed the expression of splice variants of hGHRH receptor in CAKI-1 RCC. Biodistribution studies demonstrate an in vivo uptake of (125)I-JV-1-42 by the RCC tumor tissue. The presence of specific receptor proteins that bind GHRH antagonists in CAKI-1 RCC supports the view that distinct binding sites that mediate the inhibitory effect of GHRH antagonists are present on various human cancers.
Figures
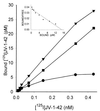
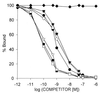
 ). VIP (♦) as well as glucagon, PACAP, JV-1–53, and PG 97–269 and other unrelated peptides, such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I, did not displace the radioligand (data not shown). One hundred percent specific binding is defined as difference between binding in absence and in presence of 10−5 M JV-1–42. Each data point represents mean of at least two experiments, done in duplicate or triplicate.
). VIP (♦) as well as glucagon, PACAP, JV-1–53, and PG 97–269 and other unrelated peptides, such as luteinizing hormone-releasing hormone, epidermal growth factor, [Tyr11]somatostatin-14, [Tyr4]bombesin, and IGF-I, did not displace the radioligand (data not shown). One hundred percent specific binding is defined as difference between binding in absence and in presence of 10−5 M JV-1–42. Each data point represents mean of at least two experiments, done in duplicate or triplicate.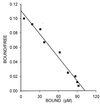


Similar articles
-
Antagonists of growth hormone-releasing hormone and vasoactive intestinal peptide inhibit tumor proliferation by different mechanisms: evidence from in vitro studies on human prostatic and pancreatic cancers.Endocrinology. 2000 Jun;141(6):2120-8. doi: 10.1210/endo.141.6.7511. Endocrinology. 2000. PMID: 10830299
-
Expression of growth hormone-releasing hormone and its receptor splice variants in human prostate cancer.J Clin Endocrinol Metab. 2002 Oct;87(10):4707-14. doi: 10.1210/jc.2002-020347. J Clin Endocrinol Metab. 2002. PMID: 12364462
-
Antagonistic actions of analogs related to growth hormone-releasing hormone (GHRH) on receptors for GHRH and vasoactive intestinal peptide on rat pituitary and pineal cells in vitro.Proc Natl Acad Sci U S A. 2000 Feb 1;97(3):1218-23. doi: 10.1073/pnas.97.3.1218. Proc Natl Acad Sci U S A. 2000. PMID: 10655511 Free PMC article.
-
GHRH and the prostate.Rev Endocr Metab Disord. 2025 Jun;26(3):467-481. doi: 10.1007/s11154-024-09922-9. Epub 2024 Nov 7. Rev Endocr Metab Disord. 2025. PMID: 39505776 Review.
-
Growth hormone-releasing hormone receptor (GHRH-R) and its signaling.Rev Endocr Metab Disord. 2025 Jun;26(3):343-352. doi: 10.1007/s11154-025-09952-x. Epub 2025 Feb 12. Rev Endocr Metab Disord. 2025. PMID: 39934495 Free PMC article. Review.
Cited by
-
Antagonists of growth hormone-releasing hormone cross the blood-brain barrier: a potential applicability to treatment of brain tumors.Proc Natl Acad Sci U S A. 2005 Aug 30;102(35):12495-500. doi: 10.1073/pnas.0504163102. Epub 2005 Aug 23. Proc Natl Acad Sci U S A. 2005. PMID: 16118272 Free PMC article.
-
Increased activity of antagonists of growth hormone-releasing hormone substituted at positions 8, 9, and 10.Proc Natl Acad Sci U S A. 2004 Feb 10;101(6):1708-13. doi: 10.1073/pnas.0307288101. Epub 2004 Jan 30. Proc Natl Acad Sci U S A. 2004. PMID: 14755056 Free PMC article.
-
The expression of growth hormone-releasing hormone (GHRH) and splice variants of its receptor in human gastroenteropancreatic carcinomas.Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11866-71. doi: 10.1073/pnas.182433099. Epub 2002 Aug 19. Proc Natl Acad Sci U S A. 2002. PMID: 12186980 Free PMC article.
-
Development of a polyclonal antiserum for the detection of the isoforms of the receptors for human growth hormone-releasing hormone on tumors.Proc Natl Acad Sci U S A. 2004 Oct 19;101(42):15160-5. doi: 10.1073/pnas.0406348101. Epub 2004 Oct 6. Proc Natl Acad Sci U S A. 2004. PMID: 15469915 Free PMC article.
-
Synergistic inhibition of growth of lung carcinomas by antagonists of growth hormone-releasing hormone in combination with docetaxel.Proc Natl Acad Sci U S A. 2006 Sep 26;103(39):14513-8. doi: 10.1073/pnas.0605309103. Epub 2006 Sep 18. Proc Natl Acad Sci U S A. 2006. PMID: 16983095 Free PMC article.
References
-
- Schally A V, Comaru-Schally A M. In: Cancer Medicine. 5th Ed. Holland J F, Frei E III, Bast R C Jr, Kufe D W, Pollock R E, Weichselbaum R R, editors. Ontario: Decker; 2000. pp. 715–729.
-
- Schally A V, Kovacs M, Toth K, Comaru-Schally A M. In: Growth Hormone Secretagogues in Clinical Practice. Bercu B B, Walker R F, editors. New York: Dekker; 1998. pp. 145–162.
-
- Schally A V, Varga J L. Trends Endocrinol Metab. 1999;10:383–391. - PubMed
-
- Zarandi M, Kovacs M, Horvath J E, Toth K, Halmos G, Groot K, Nagy A, Kele Z, Schally A V. Peptides. 1997;18:423–430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

