NMR reveals hydrogen bonds between oxygen and distal histidines in oxyhemoglobin
- PMID: 10962034
- PMCID: PMC27028
- DOI: 10.1073/pnas.190254697
NMR reveals hydrogen bonds between oxygen and distal histidines in oxyhemoglobin
Abstract
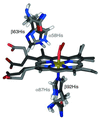
Compared with free heme, the proteins hemoglobin (Hb) and myoglobin (Mb) exhibit greatly enhanced affinity for oxygen relative to carbon monoxide. This physiologically vital property has been attributed to either steric hindrance of CO or stabilization of O(2) binding by a hydrogen bond with the distal histidine. We report here the first direct evidence of such a hydrogen bond in both alpha- and beta-chains of oxyhemoglobin, as revealed by heteronuclear NMR spectra of chain-selectively labeled samples. Using these spectra, we have assigned the imidazole ring (1)H and (15)N chemical shifts of the proximal and distal histidines in both carbonmonoxy- and oxy-Hb. Because of their proximity to the heme, these chemical shifts are extremely sensitive to the heme pocket conformation. Comparison of the measured chemical shifts with values predicted from x-ray structures suggests differences between the solution and crystal structures of oxy-Hb. The chemical shift discrepancies could be accounted for by very small displacements of the proximal and distal histidines. This suggests that NMR could be used to obtain very high-resolution heme pocket structures of Hb, Mb, and other heme proteins.
Figures

Similar articles
-
An investigation of the distal histidyl hydrogen bonds in oxyhemoglobin: effects of temperature, pH, and inositol hexaphosphate.Biochemistry. 2010 Dec 21;49(50):10606-15. doi: 10.1021/bi100927p. Epub 2010 Nov 29. Biochemistry. 2010. PMID: 21077639 Free PMC article.
-
Solution structure of carbonmonoxy myoglobin determined from nuclear magnetic resonance distance and chemical shift constraints.J Mol Biol. 1994 Nov 25;244(2):183-97. doi: 10.1006/jmbi.1994.1718. J Mol Biol. 1994. PMID: 7966330
-
Sequential assignment of proton resonances in the NMR spectrum of Zn-substituted alpha chains from human hemoglobin. Ligand-induced tertiary changes in the heme pocket.Eur J Biochem. 1993 Jun 1;214(2):383-93. doi: 10.1111/j.1432-1033.1993.tb17934.x. Eur J Biochem. 1993. PMID: 8513788
-
Spectroscopic study of Ser92 mutants of human myoglobin: hydrogen bonding effect of Ser92 to proximal His93 on structure and property of myoglobin.Biochemistry. 1994 Dec 20;33(50):14986-92. doi: 10.1021/bi00254a006. Biochemistry. 1994. PMID: 7999755
-
A myoglobin mutant designed to mimic the oxygen-avid Ascaris suum hemoglobin: elucidation of the distal hydrogen bonding network by solution NMR.Biophys J. 1997 Aug;73(2):1019-30. doi: 10.1016/S0006-3495(97)78135-9. Biophys J. 1997. PMID: 9251819 Free PMC article.
Cited by
-
Myoglobin-CO conformational substate dynamics: 2D vibrational echoes and MD simulations.Biophys J. 2002 Jun;82(6):3277-88. doi: 10.1016/S0006-3495(02)75669-5. Biophys J. 2002. PMID: 12023251 Free PMC article.
-
Distal histidine stabilizes bound O2 and acts as a gate for ligand entry in both subunits of adult human hemoglobin.J Biol Chem. 2010 Mar 19;285(12):8840-54. doi: 10.1074/jbc.M109.053934. Epub 2010 Jan 15. J Biol Chem. 2010. PMID: 20080971 Free PMC article.
-
New look at hemoglobin allostery.Chem Rev. 2015 Feb 25;115(4):1702-24. doi: 10.1021/cr500495x. Epub 2015 Jan 21. Chem Rev. 2015. PMID: 25607981 Free PMC article. Review. No abstract available.
-
A comparative NMR study of the polypeptide backbone dynamics of hemoglobin in the deoxy and carbonmonoxy forms.Biochemistry. 2007 Jun 12;46(23):6795-803. doi: 10.1021/bi602654u. Epub 2007 May 12. Biochemistry. 2007. PMID: 17497935 Free PMC article.
-
Unlocking the binding and reaction mechanism of hydroxyurea substrates as biological nitric oxide donors.J Chem Inf Model. 2012 May 25;52(5):1288-97. doi: 10.1021/ci300035c. Epub 2012 May 9. J Chem Inf Model. 2012. PMID: 22519847 Free PMC article.
References
-
- Ackers G K. Adv Protein Chem. 1998;51:185–253. - PubMed
-
- Shibayama N, Moromoto H, Saigo S. Biochemistry. 1998;37:6221–6228. - PubMed
-
- Perutz M F, Wilkinson A J, Paoli M, Dodson G G. Annu Rev Biophys Biomol Struct. 1998;27:1–34. - PubMed
-
- Eaton W A, Henry E R, Hoffrichter J, Mozzarelli A. Nat Struct Biol. 1999;6:351–358. - PubMed
-
- Ho C, Lukin J A. Embryonic Encyclopedia of Life Sciences. London: Macmillan Reference; 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

