Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development
- PMID: 10963668
- PMCID: PMC34553
- DOI: 10.1073/pnas.97.18.10050
Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development
Abstract
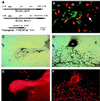
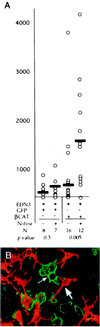
Wnt1 signaling has been implicated as one factor involved in neural crest-derived melanocyte (NC-M) development. Mice deficient for both Wnt1 and Wnt3a have a marked deficiency in trunk neural crest derivatives including NC-Ms. We have used cell lineage-directed gene targeting of Wnt signaling genes to examine the effects of Wnt signaling in mouse neural crest development. Gene expression was directed to cell lineages by infection with subgroup A avian leukosis virus vectors in lines of transgenic mice that express the retrovirus receptor tv-a. Transgenic mice with tva in either nestin-expressing neural precursor cells (line Ntva) or dopachrome tautomerase (DCT)-expressing melanoblasts (line DCTtva) were analyzed. We overstimulated Wnt signaling in two ways: directed gene transfer of Wnt1 to Ntva(+) cells and transfer of beta-catenin to DCTtva(+) NC-M precursor cells. In both methods, NC-M expansion and differentiation were effected. Significant increases were observed in the number of NC-Ms [melanin(+) and tyrosinase-related protein 1 (TYRP1)(+) cells], the differentiation of melanin(-) TYRP1(+) cells to melanin(+) TYRP1(+) NC-Ms, and the intensity of pigmentation per NC-M. These data are consistent with Wnt1 signaling being involved in both expansion and differentiation of migrating NC-Ms in the developing mouse embryo. The use of lineage-directed gene targeting will allow the dissection of signaling molecules involved in NC development and is adaptable to other mammalian developmental systems.
Figures



References
-
- Cadigan K M, Nusse R. Genes Dev. 1997;11:3286–3305. - PubMed
-
- Ikeya M, Lee S M, Johnson J E, McMahon A P, Takada S. Nature (London) 1997;389:966–970. - PubMed
-
- Dorsky R I, Moon R T, Raible D W. Nature (London) 1998;396:370–373. - PubMed
-
- Henion P D, Weston J A. Development. 1997;124:4351–4359. - PubMed
-
- Stemple D L, Anderson D J. Cell. 1992;71:973–985. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

