Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis
- PMID: 10963675
- PMCID: PMC27750
- DOI: 10.1073/pnas.97.18.10132
Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis
Abstract
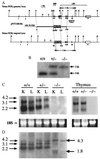
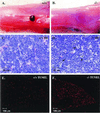
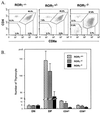
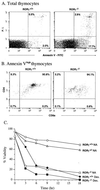
To identify the physiological functions of the retinoid-related orphan receptor gamma (RORgamma), a member of the nuclear receptor superfamily, mice deficient in RORgamma function were generated by targeted disruption. RORgamma(-/-) mice lack peripheral and mesenteric lymph nodes and Peyer's patches, indicating that RORgamma expression is indispensable for lymph node organogenesis. Although the spleen is enlarged, its architecture is normal. The number of peripheral blood CD3(+) and CD4(+) lymphocytes is reduced 6- and 10-fold, respectively, whereas the number of circulating B cells is normal. The thymus of RORgamma(-/-) mice contains 74.4% +/- 8.9% fewer thymocytes than that of wild-type mice. Flow cytometric analysis showed a decrease in the CD4(+)CD8(+) subpopulation. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining demonstrated a 4-fold increase in apoptotic cells in the cortex of the thymus of RORgamma(-/-) mice. The latter was supported by the observed increase in annexin V-positive cells. RORgamma(-/-) thymocytes placed in culture exhibit a dramatic increase in the rate of "spontaneous" apoptosis. This increase is largely associated with CD4(+)CD8(+) thymocytes and may, at least in part, be related to the greatly reduced level of expression of the anti-apoptotic gene Bcl-X(L). Flow cytometric analysis demonstrated a 6-fold rise in the percentage of cells in the S phase of the cell cycle among thymocytes from RORgamma(-/-) mice. Our observations indicate that RORgamma is essential for lymphoid organogenesis and plays an important regulatory role in thymopoiesis. Our findings support a model in which RORgamma negatively controls apoptosis in thymocytes.
Figures

References
-
- Willy P J, Mangelsdorf D J. In: Hormones and Signaling. O'Malley B W, editor. Vol. 1. San Diego: Academic; 1998. pp. 308–358.
-
- Kumar R, Thompson E B. Steroids. 1999;64:310–319. - PubMed
-
- McKenna N J, Xu J, Nawaz Z, Tsai S Y, Tsai M J, O'Malley B W. J Steroid Biochem Mol Biol. 1999;69:3–12. - PubMed
-
- Xu L, Glass C K, Rosenfeld M G. Curr Opin Genet Dev. 1999;9:140–147. - PubMed
-
- Giguere V, Tini M, Flock G, Ong E, Evans R M, Otulakowski G. Genes Dev. 1994;8:538–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

