Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment
- PMID: 10963683
- PMCID: PMC27829
- DOI: 10.1073/pnas.97.18.10231
Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment
Abstract
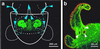
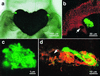
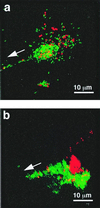
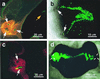
While most animal-bacterial symbioses are reestablished each successive generation, the mechanisms by which the host and its potential microbial partners ensure tissue colonization remain largely undescribed. We used the model association between the squid Euprymna scolopes and Vibrio fischeri to examine this process. This light organ symbiosis is initiated when V. fischeri cells present in the surrounding seawater enter pores on the surface of the nascent organ and colonize deep epithelia-lined crypts. We discovered that when newly hatched squid were experimentally exposed to natural seawater, the animals responded by secreting a viscous material from the pores of the organ. Animals maintained in filtered seawater produced no secretions unless Gram-negative bacteria, either living or dead, were reintroduced. The viscous material bound only lectins that are specific for either N-acetylneuraminic acid or N-acetylgalactosamine, suggesting that it was composed of a mucus-containing matrix. Complex ciliated fields on the surface of the organ produced water currents that focused the matrix into a mass that was tethered to, and suspended above, the light organ pores. When V. fischeri cells were introduced into the seawater surrounding the squid, the bacteria were drawn into its fluid-filled body cavity during ventilation and were captured in the matrix. After residing as an aggregate for several hours, the symbionts migrated into the pores and colonized the crypt epithelia. This mode of infection may be an example of a widespread strategy by which aquatic hosts increase the likelihood of successful colonization by rarely encountered symbionts.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

