Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins
- PMID: 10964959
- PMCID: PMC6772945
- DOI: 10.1523/JNEUROSCI.20-17-06540.2000
Netrin-G1: a novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins
Abstract
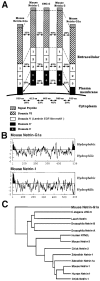
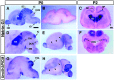
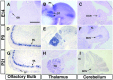
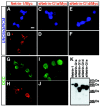
UNC-6/netrins compose a small phylogenetically conserved family of proteins that act as axon guidance cues. With a signal sequence trap method, we isolated a cDNA encoding a novel member of the UNC-6/netrin family, which we named netrin-G1. Unlike classical netrins, netrin-G1 consists of at least six isoforms of which five were predominantly anchored to the plasma membrane via glycosyl phosphatidyl-inositol linkages. Netrin-G1 transcripts were first detected in midbrain and hindbrain regions by embryonic day 12 and reached highest levels at perinatal stages in various brain regions, including olfactory bulb mitral cells, thalamus, and deep cerebellar nuclei. Its expression was primarily restricted to the CNS. Interestingly, netrin-G1 proteins did not show appreciable affinity to any netrin receptor examined. Unlike netrin-1, a secreted form of netrin-G1 consistently failed to attract circumferentially growing axons from the cerebellar plate. Our findings suggest that netrin-G1 and its putative receptors have coevolved independently from the classical netrins. The expression pattern of netrin-G1 and its predicted neuronal membrane localization suggest it may also have novel signaling functions in nervous system development.
Figures





References
-
- Ackerman SL, Kozak LP, Przyborski SA, Rund LA, Boyer BB, Knowles BB. The mouse rostral cerebellar malformation gene encodes an UNC-5-like protein. Nature. 1997;386:838–842. - PubMed
-
- Altman J, Bayer SA. Afferent and efferent connections of the cerebellar deep nuclei. In: Altman J, Bayer SA, editors. Development of the cerebellar system. CRC; Boca Raton, FL: 1997. pp. 66–79.
-
- Araujo M, Piedra ME, Herrera MT, Ros MA, Nieto MA. The expression and regulation of chick EphA7 suggests roles in limb patterning and innervation. Development. 1998;125:4195–4204. - PubMed
-
- Beck K, Hunter I, Engel J. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 1990;4:148–160. - PubMed
-
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993;5:349–354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
