Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system
- PMID: 10964967
- PMCID: PMC6772986
- DOI: 10.1523/JNEUROSCI.20-17-06619.2000
Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system
Abstract
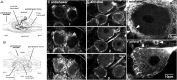
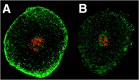
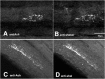
The patterned activity generated by the pyloric circuit in the stomatogastric ganglion of the spiny lobster, Panulirus interruptus, results not only from the synaptic connectivity between the 14 component neurons but also from differences in the intrinsic properties of the neurons. Presumably, differences in the complement and distribution of expressed ion channels endow these neurons with many of their distinct attributes. Each pyloric cell type possesses a unique, modulatable transient potassium current, or A-current (I(A)), that is instrumental in determining the output of the network. Two genes encode A-channels in this system, shaker and shal. We examined the hypothesis that cell-specific differences in shaker and shal channel distribution contribute to diversity among pyloric neurons. We found a stereotypic distribution of channels in the cells, such that each channel type could contribute to different aspects of the firing properties of a cell. Shal is predominantly found in the somatodendritic compartment in which it influences oscillatory behavior and spike frequency. Shaker channels are exclusively localized to the membranes of the distal axonal compartments and most likely affect distal spike propagation. Neither channel is detectably inserted into the preaxonal or proximal portions of the axonal membrane. Both channel types are targeted to synaptic contacts at the neuromuscular junction. We conclude that the differential targeting of shaker and shal to different compartments is conserved among all the pyloric neurons and that the channels most likely subserve different functions in the neuron.
Figures






References
-
- Alonso G, Widmer H. Clustering of KV4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77:617–621. - PubMed
-
- Atwood HL, Govind CK, Jahromi SS. Excitatory synapses of blue crab gastric mill muscles. Cell Tissue Res. 1977;177:145–158. - PubMed
-
- Atwood HL, Govind CK, Kwan I. Nonhomogeneous excitatory synapses of a crab stomach muscle. J Neurobiol. 1978;9:17–28. - PubMed
-
- Baldwin DH, Graubard K. Distribution of fine neurites of stomatogastric neurons of the crab Cancer borealis: evidence for a structured neuropil. J Comp Neurol. 1995;356:355–367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
