Neuronal and glial membrane potentials during sleep and paroxysmal oscillations in the neocortex
- PMID: 10964970
- PMCID: PMC6772987
- DOI: 10.1523/JNEUROSCI.20-17-06648.2000
Neuronal and glial membrane potentials during sleep and paroxysmal oscillations in the neocortex
Abstract
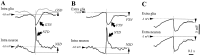
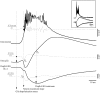
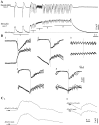
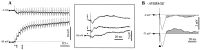
This study investigated the fluctuations in the membrane potential of cortical neurons and glial cells during the slow sleep oscillation and spike-wave (SW) seizures. We performed dual neuron-glia intracellular recordings together with multisite field potential recordings from cortical suprasylvian association areas 5 and 7 of cats under ketamine-xylazine anesthesia. Electrical stimuli applied to the cortex elicited responses consisting of a biphasic depolarization in glial cells, which was associated with an EPSP-IPSP sequence in neurons. During the slow (<1 Hz) oscillation, extracellular measurements of the potassium concentration revealed periodic increases with an amplitude of 1-2 mm, similar in shape to glial activities. We suggest that, through their uptake mechanisms, glia cells modulate the neuronal excitability and contribute to the pacing of the slow oscillation. The slow oscillation often evolved into SW paroxysms, mimicking sleep-triggered seizures. This transition was associated with increased coupling between the depolarizing events in neurons and glial cells. During seizures, the glial membrane potential displayed phasic negative events related to the onset of the paroxysmal depolarizing shifts in neurons. These events were not voltage dependent and increased their incidence and amplitude with the development of the seizure. It is suggested that the intraglial transient negativities represent field reflections of synchronized neuronal potentials. We propose that the mechanisms underlying the neuron-glia communication include, besides the traditional neurotransmitter- and ion-mediated pathways, field effects crossing their membranes as a function of the state of the cortical network.
Figures









References
-
- Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep EEG. Neuroscience. 1997;81:213–222. - PubMed
-
- Ammann D. Ion selective microelectrodes. Springer; Berlin: 1986.
-
- Amzica F, Neckelmann D. Membrane capacitance of cortical neurons and glia during sleep oscillations and spike-wave seizures. J Neurophysiol. 1999;82:2731–2746. - PubMed
-
- Amzica F, Steriade M. Short- and long-range neuronal synchronization of the slow (<1 Hz) cortical oscillation. J Neurophysiol. 1995;75:20–38. - PubMed
-
- Amzica F, Steriade M. The K-complex: Its slow (<1 Hz) rhythmicity and relation to delta waves. Neurology. 1997;49:952–959. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
