Nonrenewal statistics of electrosensory afferent spike trains: implications for the detection of weak sensory signals
- PMID: 10964972
- PMCID: PMC6772956
- DOI: 10.1523/JNEUROSCI.20-17-06672.2000
Nonrenewal statistics of electrosensory afferent spike trains: implications for the detection of weak sensory signals
Abstract
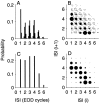
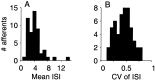
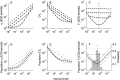
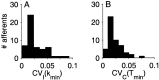
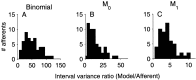
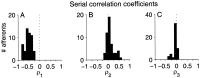
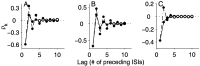
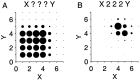
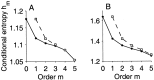
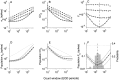
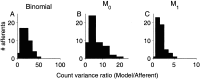
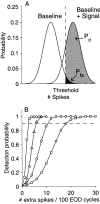
The ability of an animal to detect weak sensory signals is limited, in part, by statistical fluctuations in the spike activity of sensory afferent nerve fibers. In weakly electric fish, probability coding (P-type) electrosensory afferents encode amplitude modulations of the fish's self-generated electric field and provide information necessary for electrolocation. This study characterizes the statistical properties of baseline spike activity in P-type afferents of the brown ghost knifefish, Apteronotus leptorhynchus. Short-term variability, as measured by the interspike interval (ISI) distribution, is moderately high with a mean ISI coefficient of variation of 44%. Analysis of spike train variability on longer time scales, however, reveals a remarkable degree of regularity. The regularizing effect is maximal for time scales on the order of a few hundred milliseconds, which matches functionally relevant time scales for natural behaviors such as prey detection. Using high-order interval analysis, count analysis, and Markov-order analysis we demonstrate that the observed regularization is associated with memory effects in the ISI sequence which arise from an underlying nonrenewal process. In most cases, a Markov process of at least fourth-order was required to adequately describe the dependencies. Using an ideal observer paradigm, we illustrate how regularization of the spike train can significantly improve detection performance for weak signals. This study emphasizes the importance of characterizing spike train variability on multiple time scales, particularly when considering limits on the detectability of weak sensory signals.
Figures

References
-
- Amassian VE, Macy J, Waller HJ, Leader HS, Swift M. Transformations of afferent activity at the cuneate nucleus. In: Gerard RW, Duyff J, editors. Information Processing in the Nervous System. Excerpta Medica Foundation; Amsterdam: 1964. pp. 235–254.
-
- Bastian J. Electrolocation. I. how the electroreceptors of Apteronotus albifrons code for moving objects and other electrical stimuli. J Comp Physiol [A] 1981;144:465–479.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
