Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo
- PMID: 10964980
- PMCID: PMC6772971
- DOI: 10.1523/JNEUROSCI.20-17-06734.2000
Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo
Abstract
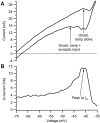
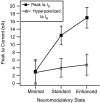
The impact of neuromodulators on active dendritic conductances was investigated by the use of intracellular recording techniques in spinal motoneurons in the adult cat. The well known lack of voltage control of dendritic regions during voltage clamp applied at the soma was used to estimate dendritic amplification of a steady monosynaptic input generated by muscle spindle Ia afferents. In preparations deeply anesthetized with pentobarbital, Ia current either decreased with depolarization or underwent a modest increase at membrane potentials above -40 mV. In unanesthetized decerebrate preparations (which have tonic activity in axons originating in the brainstem and releasing serotonin or norepinephrine), active dendritic currents caused strong amplification of Ia input. In the range of -50 to -40 mV, peak Ia current was over four times as large as that in the pentobarbital-anesthetized preparations. Exogenous administration of a noradrenergic agonist in addition to the tonic activity further enhanced amplification (sixfold increase). Amplification was not seen in preparations with spinal transections. Overall, the dendritic amplification with moderate or strong neuromodulatory drive was estimated to be large enough to allow the motoneurons innervating slow muscle fibers to be driven to their maximum force levels by remarkably small synaptic inputs. In these cells, the main role of synaptic input may be to control the activation of a highly excitable dendritic tree. The neuromodulatory control of synaptic amplification provides motor commands with the potential to adjust the level of amplification to suit the demands of different motor tasks.
Figures




Similar articles
-
Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo.J Neurophysiol. 1996 Sep;76(3):2107-10. doi: 10.1152/jn.1996.76.3.2107. J Neurophysiol. 1996. PMID: 8890322
-
Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo.J Neurophysiol. 2003 Jan;89(1):27-39. doi: 10.1152/jn.00137.2002. J Neurophysiol. 2003. PMID: 12522157
-
Amplification and linear summation of synaptic effects on motoneuron firing rate.J Neurophysiol. 2001 Jan;85(1):43-53. doi: 10.1152/jn.2001.85.1.43. J Neurophysiol. 2001. PMID: 11152704
-
Active conductances in motoneuron dendrites enhance movement capabilities.Exerc Sport Sci Rev. 2003 Apr;31(2):96-101. doi: 10.1097/00003677-200304000-00008. Exerc Sport Sci Rev. 2003. PMID: 12715974 Review.
-
Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons.Brain Res Brain Res Rev. 2002 Oct;40(1-3):1-8. doi: 10.1016/s0165-0173(02)00183-2. Brain Res Brain Res Rev. 2002. PMID: 12589901 Review.
Cited by
-
Inhibition linearizes firing rate responses in human motor units: implications for the role of persistent inward currents.J Physiol. 2017 Jan 1;595(1):179-191. doi: 10.1113/JP272823. Epub 2016 Sep 20. J Physiol. 2017. PMID: 27470946 Free PMC article.
-
Metabotropic modulation of motoneurons by scratch-like spinal network activity.J Neurosci. 2003 Sep 24;23(25):8625-9. doi: 10.1523/JNEUROSCI.23-25-08625.2003. J Neurosci. 2003. PMID: 14507961 Free PMC article.
-
A muscle-activity-dependent gain between motor cortex and EMG.J Neurophysiol. 2019 Jan 1;121(1):61-73. doi: 10.1152/jn.00329.2018. Epub 2018 Oct 31. J Neurophysiol. 2019. PMID: 30379603 Free PMC article.
-
Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis.Clin Neurophysiol. 2010 Oct;121(10):1669-79. doi: 10.1016/j.clinph.2009.12.041. Epub 2010 May 11. Clin Neurophysiol. 2010. PMID: 20462789 Free PMC article. Review.
-
Effect of caffeine on self-sustained firing in human motor units.J Physiol. 2002 Dec 1;545(2):671-9. doi: 10.1113/jphysiol.2002.025064. J Physiol. 2002. PMID: 12456842 Free PMC article. Clinical Trial.
References
-
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998a;80:2023–2037. - PubMed
-
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998b;80:2038–2045. - PubMed
-
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of physiology. Exercise: regulation and integration of multiple systems. Oxford UP; New York: 1996. pp. 1–53.
-
- Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J Neurophysiol. 1997;78:3371–3385. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
