Production and consumption of nitric oxide by three methanotrophic bacteria
- PMID: 10966405
- PMCID: PMC92235
- DOI: 10.1128/AEM.66.9.3891-3897.2000
Production and consumption of nitric oxide by three methanotrophic bacteria
Abstract
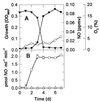
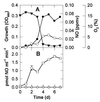
We studied nitrogen oxide production and consumption by methanotrophs Methylobacter luteus (group I), Methylosinus trichosporium OB3b (group II), and an isolate from a hardwood swamp soil, here identified by 16S ribosomal DNA sequencing as Methylobacter sp. strain T20 (group I). All could consume nitric oxide (nitrogen monoxide, NO), and produce small amounts of nitrous oxide (N(2)O). Only Methylobacter strain T20 produced large amounts of NO (>250 parts per million by volume [ppmv] in the headspace) at specific activities of up to 2.0 x 10(-17) mol of NO cell(-1) day(-1), mostly after a culture became O(2) limited. Production of NO by strain T20 occurred mostly in nitrate-containing medium under anaerobic or nearly anaerobic conditions, was inhibited by chlorate, tungstate, and O(2), and required CH(4). Denitrification (methanol-supported N(2)O production from nitrate in the presence of acetylene) could not be detected and thus did not appear to be involved in the production of NO. Furthermore, cd(1) and Cu nitrite reductases, NO reductase, and N(2)O reductase could not be detected by PCR amplification of the nirS, nirK, norB, and nosZ genes, respectively. M. luteus and M. trichosporium produced some NO in ammonium-containing medium under aerobic conditions, likely as a result of methanotrophic nitrification and chemical decomposition of nitrite. For Methylobacter strain T20, arginine did not stimulate NO production under aerobiosis, suggesting that NO synthase was not involved. We conclude that strain T20 causes assimilatory reduction of nitrate to nitrite, which then decomposes chemically to NO. The production of NO by methanotrophs such as Methylobacter strain T20 could be of ecological significance in habitats near aerobic-anaerobic interfaces where fluctuating O(2) and nitrate availability occur.
Figures



References
-
- Amaral J A, Knowles R. Growth of methanotrophs in methane and oxygen counter gradients. FEMS Microbiol Lett. 1995;126:215–220.
-
- Baumgärtner M, Koschorreck M, Conrad R. Oxidative consumption of nitric oxide by heterotrophic bacteria in soil. FEMS Microbiol Ecol. 1996;19:165–170.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

