Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms
- PMID: 10970423
- PMCID: PMC2270070
- DOI: 10.1111/j.1469-7793.2000.00203.x
Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms
Abstract
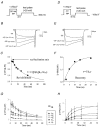
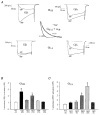
Using transient calcium phosphate transfection into the human embryonic kidney tsa-201 cell line and subsequent whole-cell patch-clamp protocols, we examined the tonic modulation of cloned N- and P/Q-type calcium channels by five different G protein beta subunits via strong depolarizing voltage prepulses. For N- and P/Q-type channels, the magnitude of inhibition was dependent on the Gbeta subtype co-expressed. Both the absolute and relative magnitudes of Gbeta subunit-induced inhibition of P/Q-type channels differed from those observed with the N-type channel. For each calcium channel subtype, kinetics of both the prepulse-mediated recovery from inhibition and the re-inhibition following the prepulse were examined for each of the Gbeta subunits by varying either the duration between the pre- and the test pulse or the length of the prepulse. For each channel subtype, we observed a differential Gbeta subunit rank order with regard to the rates of re-inhibition and recovery from inhibition. On average, P/Q-type channels exhibited more rapid rates of recovery from inhibition than those observed with N-type channels. Different Gbeta subtypes mediated different degrees of slowing of activation kinetics. The differential modulation of P/Q- and N-type channels by various Gbeta subtypes may provide a mechanism for fine tuning the amount of calcium entering the presynaptic nerve termini.
Figures


References
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage-dependence. Nature. 1989;340:153–155. - PubMed
-
- Bertram R, Behan M. Implications of G-protein-mediated Ca2+ channel inhibition for neurotransmitter release and facilitation. Journal of Computational Neuroscience. 1999;7:197–211. - PubMed
-
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nature Neuroscience. 1999;2:407–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

