Single-channel analysis of an NMDA receptor possessing a mutation in the region of the glutamate binding site
- PMID: 10970425
- PMCID: PMC2270066
- DOI: 10.1111/j.1469-7793.2000.00225.x
Single-channel analysis of an NMDA receptor possessing a mutation in the region of the glutamate binding site
Abstract
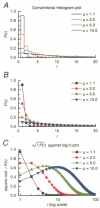
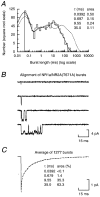
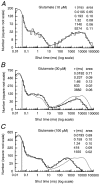
Recombinant NR1a/NR2A(T671A) N-methyl-D-aspartate (NMDA) receptor-channels, which carry a point mutation in the putative glutamate binding site that reduces glutamate potency by around 1000-fold, have been expressed in Xenopus laevis oocytes and their single-channel properties examined using patch-clamp recording techniques. Shut time distributions of channel activity were fitted with a mixture of five exponential components. The first three components in each distribution were considered to occur within a channel activation as they exhibited little or no dependence on agonist concentration. Bursts of single-channel openings were defined by a critical gap length with a mean of 5.65 +/- 0.37 ms. Shut intervals with a duration longer than this value were considered to occur between separate bursts of channel openings. Distributions of the lengths of bursts of openings were fitted with a mixture of four exponential components. The longest two components carried the majority of the charge transfer in the channel recordings and had means of 7.71 +/- 1.1 and 37.7 +/- 4.3 ms. The overall probability of a channel being open during a burst was high (mean 0.92 +/- 0.01). Brief concentration jumps (1 ms) of 10 mM glutamate were applied to outside-out patches so that a comparison between the macroscopic current relaxation and steady-state single-channel activity evoked by glutamate could be made. The decay of such macroscopic currents was fitted with a single exponential component with a mean of 32.0 +/- 3.53 ms. The good agreement between macroscopic current decay following brief agonist exposure and the value for the slowest component of the burst length distribution suggests that the bursts of openings that we identified in steady-state recordings represent individual activations of recombinant NR1a/NR2A(T671A) NMDA receptor-channels. A new way of displaying geometric distributions is suggested, and the utility of a modified definition of the 'probability of being open within a burst' is discussed. The single-channel data that we present in this paper support further the idea that the point mutation T671A in the NR2A NMDA receptor subunit affects mainly the ability of glutamate to remain bound to these channels.
Figures




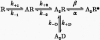
References
-
- Anson LC, Schoepfer R, Colquhoun D, Wyllie DJA. Single-channel current properties of NMDA receptor-channels, expressed in Xenopus oocytes, that contain a mutation in the glutamate binding site. The Journal of Physiology. 1999;521.P:90P.
-
- Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
-
- Béhé P, Colquhoun D, Wyllie DJA. Activation of single AMPA- and NMDA-type glutamate-receptors channels. In: Jonas P, Monyer H, editors. Handbook of Experimental Pharmacology. Vol. 141. Berlin: Springer Verlag; 1999. pp. 175–218.
-
- Béhé P, Stern P, Wyllie DJA, Nassar M, Schoepfer R, Colquhoun D. Determination of the NMDA NR1 subunit copy number in recombinant NMDA receptors. Proceedings of the Royal Society B. 1995;262:205–213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

