Structure of the ArsA ATPase: the catalytic subunit of a heavy metal resistance pump
- PMID: 10970874
- PMCID: PMC302053
- DOI: 10.1093/emboj/19.17.4838
Structure of the ArsA ATPase: the catalytic subunit of a heavy metal resistance pump
Abstract
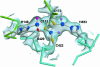
Active extrusion is a common mechanism underlying detoxification of heavy metals, drugs and antibiotics in bacteria, protozoa and mammals. In Escherichia coli, the ArsAB pump provides resistance to arsenite and antimonite. This pump consists of a soluble ATPase (ArsA) and a membrane channel (ArsB). ArsA contains two nucleotide-binding sites (NBSs) and a binding site for arsenic or antimony. Binding of metalloids stimulates ATPase activity. The crystal structure of ArsA reveals that both NBSs and the metal-binding site are located at the interface between two homologous domains. A short stretch of residues connecting the metal-binding site to the NBSs provides a signal transduction pathway that conveys information on metal occupancy to the ATP hydrolysis sites. Based on these structural features, we propose that the metal-binding site is involved directly in the process of vectorial translocation of arsenite or antimonite across the membrane. The relative positions of the NBS and the inferred mechanism of allosteric activation of ArsA provide a useful model for the interaction of the catalytic domains in other transport ATPases.
Figures

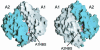


References
-
- Ambudkar S.V., Dey,S., Hrycyna,C.A., Ramachandra,M., Pastan,I. and Gottesman,M.M. (1999) Biochemical, cellular and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol., 39, 361–398. - PubMed
-
- Bhattacharjee H. and Rosen,B.P. (1996) Spatial proximity of Cys113, Cys172 and Cys422 in the metalloactivation domain of the ArsA ATPase. J. Biol. Chem., 271, 24465–24470. - PubMed
-
- Bhattacharjee H., Li,J., Ksenzenko,M.Y. and Rosen,B.P. (1995) Role of cysteinyl residues in metalloactivation of the oxyanion-translocating ArsA ATPase. J. Biol. Chem., 270, 11245–11250. - PubMed
-
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

