Steady-state pharmacokinetics of lithium in healthy volunteers receiving concomitant meloxicam
- PMID: 10971303
- PMCID: PMC2014978
- DOI: 10.1046/j.1365-2125.2000.00241.x
Steady-state pharmacokinetics of lithium in healthy volunteers receiving concomitant meloxicam
Abstract
Aims: This open, controlled study investigated the effect of concomitant 15 mg oral meloxicam on the pharmacokinetics of lithium in healthy male volunteers.
Methods: On days 1-14 lithium was coadministered with meloxicam to 16 volunteers; on days 10-14 lithium was administered in individualized dosage regimes to achieve stable lithium plasma concentrations in the lower therapeutic range of 0.3-0.7 mmol l(-1). A 12 h steady-state concentration profile for lithium was obtained at day 14, after which meloxicam was withdrawn. The lithium dose remained unchanged from day 15 to day 22, at which time a second lithium concentration profile was determined.
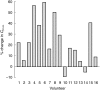
Results: Lithium and meloxicam were well tolerated throughout the study and all 16 volunteers completed the study. Lithium predose concentrations (Cpre,ss) and area under the curve (AUCss) values both increased by 21% (paired t-test P = 0.0002; 90% confidence intervals for test/reference ratios: 113-130% and 115-128%, respectively) when lithium was coadministered with meloxicam compared with values obtained for lithium alone. The geometric mean lithium Cpre,ss was 0.65 mmol l(-1) when coadministered with meloxicam and 0.54 mmol l(-1) for lithium alone. Lithium Cmax,ss values were increased by 16% by coadministration of meloxicam, from 0.97 mmol l(-1) to 1.12 mmol l(-1). The total plasma clearance of lithium was lower with concomitant meloxicam administration (82.5% of value for lithium alone).
Conclusions: Meloxicam (15 mg) moderately increased the plasma concentration of lithium in healthy volunteers, but by a magnitude thought to be of low clinical relevance. Nevertheless, lithium plasma concentrations should be closely monitored in patients receiving concomitant meloxicam and lithium therapy.
Figures


Similar articles
-
A comparison of the effects of nabumetone vs meloxicam on serum thromboxane B2 and platelet function in healthy volunteers.Br J Clin Pharmacol. 2002 Jun;53(6):644-7. doi: 10.1046/j.1365-2125.2002.01605.x. Br J Clin Pharmacol. 2002. PMID: 12047490 Free PMC article. Clinical Trial.
-
Lack of interaction between meloxicam and warfarin in healthy volunteers.Eur J Clin Pharmacol. 1997;51(5):421-5. doi: 10.1007/s002280050224. Eur J Clin Pharmacol. 1997. PMID: 9049585
-
Limitation of the in vitro whole blood assay for predicting the COX selectivity of NSAIDs in clinical use.Br J Clin Pharmacol. 2002 Mar;53(3):255-65. doi: 10.1046/j.0306-5251.2001.01533.x. Br J Clin Pharmacol. 2002. PMID: 11874389 Free PMC article. Clinical Trial.
-
[Meloxicam (Mobic): a review of its pharmacological and clinical profile].Nihon Yakurigaku Zasshi. 2002 Dec;120(6):391-7. doi: 10.1254/fpj.120.391. Nihon Yakurigaku Zasshi. 2002. PMID: 12528470 Review. Japanese.
-
Meloxicam: a reappraisal of pharmacokinetics, efficacy and safety.Expert Opin Pharmacother. 2005 Oct;6(12):2117-40. doi: 10.1517/14656566.6.12.2117. Expert Opin Pharmacother. 2005. PMID: 16197363 Review.
Cited by
-
Lithium in Paediatric Patients with Bipolar Disorder: Implications for Selection of Dosage Regimens via Population Pharmacokinetics/Pharmacodynamics.Clin Pharmacokinet. 2017 Jan;56(1):77-90. doi: 10.1007/s40262-016-0430-3. Clin Pharmacokinet. 2017. PMID: 27393139 Clinical Trial.
-
First-dose pharmacokinetics of lithium carbonate in children and adolescents.J Clin Psychopharmacol. 2010 Aug;30(4):404-10. doi: 10.1097/JCP.0b013e3181e66a62. J Clin Psychopharmacol. 2010. PMID: 20531219 Free PMC article.
-
Population Pharmacokinetics and Exposure-Response of Lithium Carbonate in Patients Based on Tubular Reabsorption Mechanisms.Eur J Drug Metab Pharmacokinet. 2019 Jun;44(3):329-338. doi: 10.1007/s13318-018-0536-0. Eur J Drug Metab Pharmacokinet. 2019. PMID: 30536114
-
Lithium: updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring.CNS Drugs. 2009;23(4):331-49. doi: 10.2165/00023210-200923040-00005. CNS Drugs. 2009. PMID: 19374461 Review.
-
Drug Interactions with Lithium: An Update.Clin Pharmacokinet. 2016 Aug;55(8):925-41. doi: 10.1007/s40262-016-0370-y. Clin Pharmacokinet. 2016. PMID: 26936045 Review.
References
-
- Price LH, Heninger GR. Lithium in the treatment of mood disorders. N Engl J Med. 1994;331:591–598. - PubMed
-
- Ward ME, Musa MN, Bailey L. Clinical pharmacokinetics of lithium. J Clin Pharmacol. 1994;34:280. - PubMed
-
- Thornhill DP. The biological disposition and kinetics of lithium. Biopharm Drug Dispos. 1981;2:305–322. - PubMed
-
- Goodnick PJ, Fieve RR, Meltzer HL, Dunner DL. Lithium elimination half-life and duration of therapy. Clin Pharmacol Ther. 1981;29:47–50. - PubMed
-
- Finley PR, Warner MD, Peabody CA. Clinical relevance of drug interactions with lithium. Clin Pharmacokin. 1995;29:172–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

