Toll-related receptors and the control of antimicrobial peptide expression in Drosophila
- PMID: 10973475
- PMCID: PMC27057
- DOI: 10.1073/pnas.180130797
Toll-related receptors and the control of antimicrobial peptide expression in Drosophila
Abstract
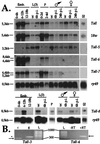
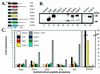
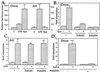
Insects defend themselves against infectious microorganisms by synthesizing potent antimicrobial peptides. Drosophila has appeared in recent years as a favorable model to study this innate host defense. A genetic analysis of the regulation of the antifungal peptide drosomycin has demonstrated a key role for the transmembrane receptor Toll, which prompted the search for mammalian homologs. Two of these, Toll-like receptor (TLR)2 and TLR4, recently were shown to play a critical role in innate immunity against bacteria. Here we describe six additional Toll-related genes (Toll-3 to Toll-8) in Drosophila in addition to 18-wheeler. Two of these genes, Toll-3 and Toll-4, are expressed at a low level. Toll-6, -7, and -8, on the other hand, are expressed at high levels during embryogenesis and molting, suggesting that, like Toll and 18w, they perform developmental functions. Finally, Toll-5 is expressed only in larvae and adults. By using chimeric constructs, we have tested the capacity of the signaling Toll/IL-1R homology domains of these receptors to activate antimicrobial peptide promoters and found that only Toll and Toll-5 can activate the drosomycin promoter in transfected cells, thus demonstrating specificity at the level of the Toll/IL-1R homology domain. In contrast, none of these constructs activated antibacterial peptide promoters, suggesting that Toll-related receptors are not involved in the regulation of antibacterial peptide expression. This result was independently confirmed by the demonstration that a dominant-negative version of the kinase Pelle can block induction of drosomycin by the cytokine Spaetzle, but does not affect induction of the antibacterial peptide attacin by lipopolysaccharide.
Figures
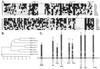
Comment in
-
Viral interference with IL-1 and toll signaling.Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):10682-3. doi: 10.1073/pnas.97.20.10682. Proc Natl Acad Sci U S A. 2000. PMID: 11005852 Free PMC article. No abstract available.
References
-
- Hoffmann J, Reichhart J. Trends Cell Biol. 1997;7:309–316. - PubMed
-
- Lemaitre B, Nicolas E, Michaut L, Reichhart J, Hoffmann J. Cell. 1996;86:973–983. - PubMed
-
- DeLotto Y, DeLotto R. Mech Dev. 1998;72:141–148. - PubMed
-
- Levashina E A, Langley E, Green C, Gubb D, Ashburner M, Hoffmann J A, Reichhart J M. Science. 1999;285:1917–1919. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

