Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes
- PMID: 10973476
- PMCID: PMC27072
- DOI: 10.1073/pnas.180143997
Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes
Abstract
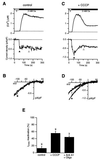
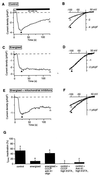
In addition to their well-known functions in cellular energy transduction, mitochondria play an important role in modulating the amplitude and time course of intracellular Ca(2+) signals. In many cells, mitochondria act as Ca(2+) buffers by taking up and releasing Ca(2+), but this simple buffering action by itself often cannot explain the organelle's effects on Ca(2+) signaling dynamics. Here we describe the functional interaction of mitochondria with store-operated Ca(2+) channels in T lymphocytes as a mechanism of mitochondrial Ca(2+) signaling. In Jurkat T cells with functional mitochondria, prolonged depletion of Ca(2+) stores causes sustained activation of the store-operated Ca(2+) current, I(CRAC) (CRAC, Ca(2+) release-activated Ca(2+)). Inhibition of mitochondrial Ca(2+) uptake by compounds that dissipate the intramitochondrial potential unmasks Ca(2+)-dependent inactivation of I(CRAC). Thus, functional mitochondria are required to maintain CRAC-channel activity, most likely by preventing local Ca(2+) accumulation near sites that govern channel inactivation. In cells stimulated through the T-cell antigen receptor, acute blockade of mitochondrial Ca(2+) uptake inhibits the nuclear translocation of the transcription factor NFAT in parallel with CRAC channel activity and [Ca(2+)](i) elevation, indicating a functional link between mitochondrial regulation of I(CRAC) and T-cell activation. These results demonstrate a role for mitochondria in controlling Ca(2+) channel activity and signal transmission from the plasma membrane to the nucleus.
Figures

References
-
- Gunter T E, Gunter K K, Sheu S S, Gavin C E. Am J Physiol. 1994;267:C313–C339. - PubMed
-
- Hajnóczky G, Robb-Gaspers L D, Seitz M B, Thomas A P. Cell. 1995;82:415–424. - PubMed
-
- Rizzuto R, Brini M, Murgia M, Pozzan T. Science. 1993;262:744–747. - PubMed
-
- Rizzuto R, Pinton P, Carrington W, Fay F S, Fogarty K E, Lifshitz L M, Tuft R A, Pozzan T. Science. 1998;280:1763–1766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

