Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis
- PMID: 10973481
- PMCID: PMC27045
- DOI: 10.1073/pnas.180316397
Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis
Abstract
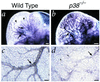
The p38 family of mitogen-activated protein kinases (MAPKs) mediates signaling in response to environmental stresses and inflammatory cytokines, but the requirements for the p38 MAPK pathway in normal mammalian development have not been elucidated. Here, we show that targeted disruption of the p38alpha MAPK gene results in homozygous embryonic lethality because of severe defects in placental development. Although chorioallantoic placentation is initiated appropriately in p38alpha null homozygotes, placental defects are manifest at 10.5 days postcoitum as nearly complete loss of the labyrinth layer and significant reduction of the spongiotrophoblast. In particular, p38alpha mutant placentas display lack of vascularization of the labyrinth layer as well as increased rates of apoptosis, consistent with a defect in placental angiogenesis. Furthermore, p38alpha mutants display abnormal angiogenesis in the embryo proper as well as in the visceral yolk sac. Thus, our results indicate a requirement for p38alpha MAPK in diploid trophoblast development and placental vascularization and suggest a more general role for p38 MAPK signaling in embryonic angiogenesis.
Figures




References
-
- Cross J C, Werb Z, Fisher S J. Science. 1994;266:1508–1518. - PubMed
-
- Risau W. Nature (London) 1997;386:671–674. - PubMed
-
- Han J, Lee J D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. - PubMed
-
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. Cell. 1994;78:1027–1037. - PubMed
-
- Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

