DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis
- PMID: 10973486
- PMCID: PMC27079
- DOI: 10.1073/pnas.180320497
DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis
Abstract
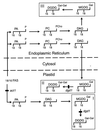
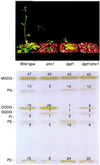
The galactolipids, mono- and digalactosyldiacylglycerol (DGDG), are the most common nonphosphorous lipids in the biosphere and account for 80% of the membrane lipids found in green plant tissues. These lipids are major constituents of photosynthetic membranes (thylakoids), and a large body of evidence suggests that galactolipids are associated primarily with plastid membranes in seed plants. A null-mutant of Arabidopsis (dgd1), which lacks the DGDG synthase (DGD1) resulting in a 90% reduction in the amount of DGDG under normal growth conditions, accumulated DGDG after phosphate deprivation up to 60% of the amount present in the wild type. This observation suggests the existence of a DGD1-independent pathway of galactolipid biosynthesis. The fatty acid composition of the newly formed DGDG was distinct, showing an enrichment of 16-carbon fatty acids in the C-1 position of the glycerol backbone of DGDG. Roots with their rudimentary plastids accumulated large amounts of DGDG after phosphate deprivation, suggesting that this galactolipid may be located in extraplastidic membranes. Corroborating evidence for this hypothesis was obtained directly by fractionation of subcellular membranes from leaf tissue and indirectly by lipid analysis of the phosphate-deprived fad3 mutant primarily deficient in extraplastidic fatty acid desaturation. The discovery of extraplastidic DGDG biosynthesis induced by phosphate deprivation has revealed a biochemical mechanism for plants to conserve phosphate. Apparently, plants replace phospholipids with nonphosphorous galactolipids if environmental conditions such as phosphate deprivation require this for survival.
Figures


References
-
- Minnikin D E, Abdolrahimzadeh H, Baddiley J. Nature (London) 1974;249:268–269. - PubMed
-
- Benning C, Huang Z H, Gage D A. Arch Biochem Biophys. 1995;317:103–111. - PubMed
-
- Güler S, Seeliger A, Härtel H, Renger G, Benning C. J Biol Chem. 1996;271:7501–7507. - PubMed
-
- Benning C. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:53–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

