Nuclei and microtubule asters stimulate maturation/M phase promoting factor (MPF) activation in Xenopus eggs and egg cytoplasmic extracts
- PMID: 10973988
- PMCID: PMC2175258
- DOI: 10.1083/jcb.150.5.963
Nuclei and microtubule asters stimulate maturation/M phase promoting factor (MPF) activation in Xenopus eggs and egg cytoplasmic extracts
Abstract
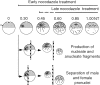
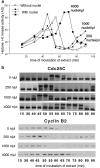
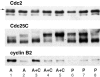
Although maturation/M phase promoting factor (MPF) can activate autonomously in Xenopus egg cytoplasm, indirect evidence suggests that nuclei and centrosomes may focus activation within the cell. We have dissected the contribution of these structures to MPF activation in fertilized eggs and in egg fragments containing different combinations of nuclei, centrosomes, and microtubules by following the behavior of Cdc2 (the kinase component of MPF), the regulatory subunit cyclin B, and the activating phosphatase Cdc25. The absence of the entire nucleus-centrosome complex resulted in a marked delay in MPF activation, whereas the absence of the centrosome alone caused a lesser delay. Nocodazole treatment to depolymerize microtubules through first interphase had an effect equivalent to removing the centrosome. Furthermore, microinjection of isolated centrosomes into anucleate eggs promoted MPF activation and advanced the onset of surface contraction waves, which are close indicators of MPF activation and could be triggered by ectopic MPF injection. Finally, we were able to demonstrate stimulation of MPF activation by the nucleus-centriole complex in vitro, as low concentrations of isolated sperm nuclei advanced MPF activation in cycling cytoplasmic extracts. Together these results indicate that nuclei and microtubule asters can independently stimulate MPF activation and that they cooperate to enhance activation locally.
Figures






Similar articles
-
Pre-M phase-promoting factor associates with annulate lamellae in Xenopus oocytes and egg extracts.Mol Biol Cell. 2003 Mar;14(3):1125-37. doi: 10.1091/mbc.e02-08-0511. Mol Biol Cell. 2003. PMID: 12631728 Free PMC article.
-
Changes in microtubule structures during the first cell cycle of physiologically polyspermic newt eggs.Mol Reprod Dev. 1997 Jun;47(2):210-21. doi: 10.1002/(SICI)1098-2795(199706)47:2<210::AID-MRD13>3.0.CO;2-3. Mol Reprod Dev. 1997. PMID: 9136124
-
Control of sperm nuclear behavior in physiologically polyspermic newt eggs: possible involvement of MPF.Dev Biol. 1990 Dec;142(2):301-12. doi: 10.1016/0012-1606(90)90351-i. Dev Biol. 1990. PMID: 2257969
-
Localised MPF regulation in eggs.Biol Cell. 2000 Jul;92(3-4):245-53. doi: 10.1016/s0248-4900(00)01067-4. Biol Cell. 2000. PMID: 11043412 Review.
-
Nuclear responses to MPF activation and inactivation in Xenopus oocytes and early embryos.Biol Cell. 1998 Nov;90(8):591-9. Biol Cell. 1998. PMID: 10069004 Review.
Cited by
-
Optimisation of an oviposition protocol employing human chorionic and pregnant mare serum gonadotropins in the barred frog Mixophyes fasciolatus (Myobatrachidae).Reprod Biol Endocrinol. 2012 Aug 21;10:60. doi: 10.1186/1477-7827-10-60. Reprod Biol Endocrinol. 2012. PMID: 22909256 Free PMC article.
-
Nuclear envelope breakdown in starfish oocytes proceeds by partial NPC disassembly followed by a rapidly spreading fenestration of nuclear membranes.J Cell Biol. 2003 Mar 31;160(7):1055-68. doi: 10.1083/jcb.200211076. Epub 2003 Mar 24. J Cell Biol. 2003. PMID: 12654902 Free PMC article.
-
Identification of the nuclear localization signal in Xenopus cyclin E and analysis of its role in replication and mitosis.Mol Biol Cell. 2002 Dec;13(12):4388-400. doi: 10.1091/mbc.e02-07-0449. Mol Biol Cell. 2002. PMID: 12475960 Free PMC article.
-
Pre-M phase-promoting factor associates with annulate lamellae in Xenopus oocytes and egg extracts.Mol Biol Cell. 2003 Mar;14(3):1125-37. doi: 10.1091/mbc.e02-08-0511. Mol Biol Cell. 2003. PMID: 12631728 Free PMC article.
-
Fertilization and preimplantation development of mouse oocytes after prolonged incubation with caffeine.Reprod Med Biol. 2004 Dec 3;3(4):245-251. doi: 10.1111/j.1447-0578.2004.00077.x. eCollection 2004 Dec. Reprod Med Biol. 2004. PMID: 29662386 Free PMC article.
References
-
- Abrieu A., Brassac T., Galas S., Fisher D., Labbé J.C., Dorée M. The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 1998;111:1751–1757. - PubMed
-
- Alfa C.E., Ducommun B., Beach D., Hyams J.S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. - PubMed
-
- Aligue R., Wu L., Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J. Biol. Chem. 1997;272:13320–13325. - PubMed
-
- Ashcroft N.R., Srayko M., Kosinski M.E., Mains P.E., Golden A. RNA-mediated interference of a cdc25 homolog in Caenorhabditis elegans results in defects in the embryonic cortical membrane, meiosis, and mitosis. Dev. Biol. 1999;206:15–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

