Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway
- PMID: 10973995
- PMCID: PMC2175246
- DOI: 10.1083/jcb.150.5.1057
Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway
Abstract
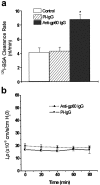
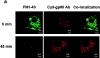
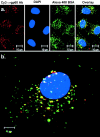
We tested the hypothesis that the albumin-docking protein gp60, which is localized in caveolae, couples to the heterotrimeric GTP binding protein G(i), and thereby activates plasmalemmal vesicle formation and the directed migration of vesicles in endothelial cells (ECs). We used the water-soluble styryl pyridinium dye N-(3-triethylaminopropyl)-4-(p-dibutylaminostyryl) pyridinium dibromide (FM 1-43) to quantify vesicle trafficking by confocal and digital fluorescence microscopy. FM 1-43 and fluorescently labeled anti-gp60 antibody (Ab) were colocalized in endocytic vesicles within 5 min of gp60 activation. Vesicles migrated to the basolateral surface where they released FM 1-43, the fluid phase styryl probe. FM 1-43 fluorescence disappeared from the basolateral EC surface without the loss of anti-gp60 Ab fluorescence. Activation of cell-surface gp60 by cross-linking (using anti-gp60 Ab and secondary Ab) in EC grown on microporous filters increased transendothelial (125)I-albumin permeability without altering liquid permeability (hydraulic conductivity), thus, indicating the dissociation of hydraulic conductivity from the albumin permeability pathway. The findings that the sterol-binding agent, filipin, prevented gp60-activated vesicle formation and that caveolin-1 and gp60 were colocalized in vesicles suggest the caveolar origin of endocytic vesicles. Pertussis toxin pretreatment and expression of the dominant negative construct encoding an 11-amino acid G(alphai) carboxyl-terminal peptide inhibited endothelial (125)I-albumin endocytosis and vesicle formation induced by gp60 activation. Expression of dominant negative Src (dn-Src) and overexpression of wild-type caveolin-1 also prevented gp60-activated endocytosis. Caveolin-1 overexpression resulted in the sequestration of G(alphai) with the caveolin-1, whereas dn-Src inhibited G(alphai) binding to caveolin-1. Thus, vesicle formation induced by gp60 and migration of vesicles to the basolateral membrane requires the interaction of gp60 with caveolin-1, followed by the activation of the downstream G(i)-coupled Src kinase signaling pathway.
Figures










Similar articles
-
Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells.J Biol Chem. 2004 Nov 12;279(46):48055-62. doi: 10.1074/jbc.M405837200. Epub 2004 Sep 2. J Biol Chem. 2004. PMID: 15345719
-
Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway.J Biol Chem. 1997 Oct 10;272(41):25968-75. doi: 10.1074/jbc.272.41.25968. J Biol Chem. 1997. PMID: 9325331
-
Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells.J Biol Chem. 2004 May 7;279(19):20392-400. doi: 10.1074/jbc.M308710200. Epub 2004 Mar 8. J Biol Chem. 2004. PMID: 15007081
-
Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function.Histochem Cell Biol. 2002 Feb;117(2):105-12. doi: 10.1007/s00418-001-0367-x. Epub 2002 Jan 22. Histochem Cell Biol. 2002. PMID: 11935286 Review.
-
The Caveolin genes: from cell biology to medicine.Ann Med. 2004;36(8):584-95. doi: 10.1080/07853890410018899. Ann Med. 2004. PMID: 15768830 Review.
Cited by
-
Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway.Genes Nutr. 2013 Mar;8(2):231-9. doi: 10.1007/s12263-012-0319-1. Epub 2012 Sep 16. Genes Nutr. 2013. PMID: 22983702 Free PMC article.
-
Energy and Dynamics of Caveolae Trafficking.Front Cell Dev Biol. 2021 Jan 21;8:614472. doi: 10.3389/fcell.2020.614472. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33692993 Free PMC article. Review.
-
Sorting it out in endosomes: an emerging concept in renal epithelial cell transport regulation.Physiology (Bethesda). 2010 Oct;25(5):280-92. doi: 10.1152/physiol.00022.2010. Physiology (Bethesda). 2010. PMID: 20940433 Free PMC article. Review.
-
GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1.J Cell Biol. 2007 May 21;177(4):717-30. doi: 10.1083/jcb.200609174. J Cell Biol. 2007. PMID: 17517964 Free PMC article.
-
Inhibition of non-receptor tyrosine kinase Src induces phosphoserine 256-independent aquaporin-2 membrane accumulation.J Physiol. 2019 Mar;597(6):1627-1642. doi: 10.1113/JP277024. Epub 2018 Dec 21. J Physiol. 2019. PMID: 30488437 Free PMC article.
References
-
- Anderson R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. - PubMed
-
- Bocci V. Efficient labeling of serum proteins with 131I using chloramine-T. Int. J. Appl. Radiat. Isot. 1964;15:449–456. - PubMed
-
- Del Vecchio P.J., Siflinger-Birnboim A., Shepard J.M., Bizios R., Cooper J.A., Malik A.B. Endothelial monolayer permeability to macromolecules. Fed. Proc. 1987;46:2511–2515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

