Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing
- PMID: 10973997
- PMCID: PMC2175240
- DOI: 10.1083/jcb.150.5.1085
Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing
Abstract
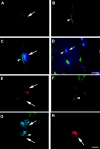
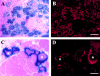
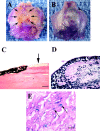
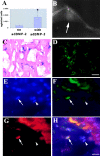
Several recent studies suggest the isolation of stem cells in skeletal muscle, but the functional properties of these muscle-derived stem cells is still unclear. In the present study, we report the purification of muscle-derived stem cells from the mdx mouse, an animal model for Duchenne muscular dystrophy. We show that enrichment of desmin(+) cells using the preplate technique from mouse primary muscle cell culture also enriches a cell population expressing CD34 and Bcl-2. The CD34(+) cells and Bcl-2(+) cells were found to reside within the basal lamina, where satellite cells are normally found. Clonal isolation and characterization from this CD34(+)Bcl-2(+) enriched population yielded a putative muscle-derived stem cell, mc13, that is capable of differentiating into both myogenic and osteogenic lineage in vitro and in vivo. The mc13 cells are c-kit and CD45 negative and express: desmin, c-met and MNF, three markers expressed in early myogenic progenitors; Flk-1, a mouse homologue of KDR recently identified in humans as a key marker in hematopoietic cells with stem cell-like characteristics; and Sca-1, a marker for both skeletal muscle and hematopoietic stem cells. Intramuscular, and more importantly, intravenous injection of mc13 cells result in muscle regeneration and partial restoration of dystrophin in mdx mice. Transplantation of mc13 cells engineered to secrete osteogenic protein differentiate in osteogenic lineage and accelerate healing of a skull defect in SCID mice. Taken together, these results suggest the isolation of a population of muscle-derived stem cells capable of improving both muscle regeneration and bone healing.
Figures






Similar articles
-
Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells.Hum Gene Ther. 2001 Apr 10;12(6):619-28. doi: 10.1089/104303401300057306. Hum Gene Ther. 2001. PMID: 11426462
-
Intraarterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice.J Cell Biol. 2001 Jan 22;152(2):335-48. doi: 10.1083/jcb.152.2.335. J Cell Biol. 2001. PMID: 11266450 Free PMC article.
-
Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice.Tissue Eng Part A. 2012 Feb;18(3-4):232-41. doi: 10.1089/ten.TEA.2010.0553. Epub 2011 Oct 11. Tissue Eng Part A. 2012. PMID: 21854253 Free PMC article.
-
A new look at the origin, function, and "stem-cell" status of muscle satellite cells.Dev Biol. 2000 Feb 15;218(2):115-24. doi: 10.1006/dbio.1999.9565. Dev Biol. 2000. PMID: 10656756 Review.
-
Stem cells in adult skeletal muscle.Trends Cardiovasc Med. 2003 Apr;13(3):123-8. doi: 10.1016/s1050-1738(03)00024-0. Trends Cardiovasc Med. 2003. PMID: 12691677 Review.
Cited by
-
Periosteal cells are a major source of soft callus in bone fracture.J Bone Miner Metab. 2013 Jul;31(4):390-8. doi: 10.1007/s00774-013-0429-x. Epub 2013 Mar 12. J Bone Miner Metab. 2013. PMID: 23475152
-
Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model.Eur Cell Mater. 2012 Jun 5;23:400-12; discussion 412. doi: 10.22203/ecm.v023a31. Eur Cell Mater. 2012. PMID: 22665162 Free PMC article.
-
The promise of stem cell therapy to restore urethral sphincter function.Curr Urol Rep. 2007 Sep;8(5):373-8. doi: 10.1007/s11934-007-0034-4. Curr Urol Rep. 2007. PMID: 17880836 Review.
-
NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis.Am J Pathol. 2005 Oct;167(4):1105-17. doi: 10.1016/S0002-9440(10)61199-6. Am J Pathol. 2005. PMID: 16192645 Free PMC article.
-
Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches.Am J Pathol. 2004 Mar;164(3):773-9. doi: 10.1016/S0002-9440(10)63165-3. Am J Pathol. 2004. PMID: 14982831 Free PMC article.
References
-
- Andrews R.G., Singer J.W., Bernstein I.D. Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986;67:842–845. - PubMed
-
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Egicjo C., Ishihara T., Nonak I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1989;333:861–863. - PubMed
-
- Barch, M.J. 1991. Chromosome analysis. In The ACT Cytogenetics Laboratory Manual. 2nd edition. Raven Press, Ltd., NY, NY. 349.
-
- Baroffio A., Hamann M., Bernheim L., Bochaton-Piallat M.L., Gabbiani G., Bader C.R. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. - PubMed
-
- Beauchamps J.R., Morgan J.E., Pagel C.N., Partridge T.A. Quantitative studies of the efficacy of myoblast transplantation. Muscle Nerve. 1994;18:S261.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

