Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride
- PMID: 10974021
- PMCID: PMC381289
- DOI: 10.1172/JCI10318
Role of p38 in the regulation of renal cortical cyclooxygenase-2 expression by extracellular chloride
Abstract
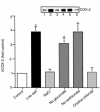
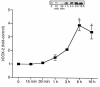
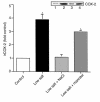
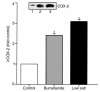
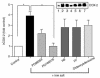
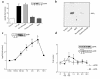
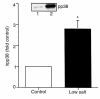
We have previously shown that in renal cortex, COX-2 expression is localized to macula densa and surrounding cortical thick ascending limb of Henle (cTALH). Dietary salt restriction increases local expression of COX-2, which mediates renin production and secretion. Given that decreased luminal chloride [Cl(-)] at the level of the macula densa increases renin production and secretion, we investigated the role of extracellular ion concentration on COX-2 expression. Quiescent rabbit cTALH cells were incubated in a physiological salt solution containing high or low levels of NaCl. Immunoreactive COX-2 expression increased significantly in the low NaCl solution. COX-2 expression also increased after administration of the Na(+)/K(+)/2Cl(-) cotransport inhibitor, bumetanide. Selective substitution of chloride led to increased COX-2 expression, whereas selective substitution of sodium had no effect. The p38 MAP kinase inhibitor PD169316 decreased low NaCl-induced COX-2 expression. Low-salt or low-chloride medium induced cultured cTALH to accumulate >/= 3-fold higher levels of pp38, the activated (phosphorylated) form of p38; low-salt medium also increased pJNK and pERK levels. Feeding rats a low-salt diet for 14 days induced a significant increase in renal cortical pp38 expression, predominantly in the macula densa and cTALH. These results suggest that reduced extracellular chloride leads to increased COX-2 expression, which may be mediated by activation of a p38-dependent signaling pathway.
Figures

Comment in
-
Ions and signal transduction in the macula densa.J Clin Invest. 2000 Sep;106(5):633-5. doi: 10.1172/JCI10930. J Clin Invest. 2000. PMID: 10974015 Free PMC article. No abstract available.
Similar articles
-
Cyclooxygenase-2 and the renal renin-angiotensin system.Acta Physiol Scand. 2004 Aug;181(4):543-7. doi: 10.1111/j.1365-201X.2004.01329.x. Acta Physiol Scand. 2004. PMID: 15283769 Review.
-
Cyclooxygenase-2 expression in cultured cortical thick ascending limb of Henle increases in response to decreased extracellular ionic content by both transcriptional and post-transcriptional mechanisms. Role of p38-mediated pathways.J Biol Chem. 2002 Nov 22;277(47):45638-43. doi: 10.1074/jbc.M206040200. Epub 2002 Sep 16. J Biol Chem. 2002. PMID: 12237297
-
Nitric oxide regulates renal cortical cyclooxygenase-2 expression.Am J Physiol Renal Physiol. 2000 Jul;279(1):F122-9. doi: 10.1152/ajprenal.2000.279.1.F122. Am J Physiol Renal Physiol. 2000. PMID: 10894794
-
Furosemide stimulates macula densa cyclooxygenase-2 expression in rats.Kidney Int. 2001 Jan;59(1):62-8. doi: 10.1046/j.1523-1755.2001.00466.x. Kidney Int. 2001. PMID: 11135058
-
Physiological regulation of cyclooxygenase-2 in the kidney.Am J Physiol Renal Physiol. 2001 Jul;281(1):F1-11. doi: 10.1152/ajprenal.2001.281.1.F1. Am J Physiol Renal Physiol. 2001. PMID: 11399641 Review.
Cited by
-
Nonsteroidal Anti-Inflammatory Drugs and the Kidney.Pharmaceuticals (Basel). 2010 Jul 21;3(7):2291-2321. doi: 10.3390/ph3072291. Pharmaceuticals (Basel). 2010. PMID: 27713354 Free PMC article. Review.
-
Macula densa sensing and signaling mechanisms of renin release.J Am Soc Nephrol. 2010 Jul;21(7):1093-6. doi: 10.1681/ASN.2009070759. Epub 2010 Apr 1. J Am Soc Nephrol. 2010. PMID: 20360309 Free PMC article.
-
Ions and signal transduction in the macula densa.J Clin Invest. 2000 Sep;106(5):633-5. doi: 10.1172/JCI10930. J Clin Invest. 2000. PMID: 10974015 Free PMC article. No abstract available.
-
Intracellular monovalent ions as second messengers.J Membr Biol. 2006 Apr;210(3):161-72. doi: 10.1007/s00232-006-0857-9. Epub 2006 Aug 14. J Membr Biol. 2006. PMID: 16909338 Review.
-
Cyclooxygenase 2 inhibition suppresses tubuloglomerular feedback: roles of thromboxane receptors and nitric oxide.Am J Physiol Renal Physiol. 2009 Apr;296(4):F790-4. doi: 10.1152/ajprenal.90446.2008. Epub 2009 Jan 14. Am J Physiol Renal Physiol. 2009. PMID: 19144694 Free PMC article.
References
-
- Persson AEG, et al. Macula densa function. Kidney Int. 1991;32(Suppl. 32):S39–S44. - PubMed
-
- Schlatter E, Salomonsson M, Persson AEG, Greger R. Macula densa cells sense luminal NaCl concentration via furosemide sensitive Na+2C1-K+ cotransport. Pflugers Arch. 1989;414:286–290. - PubMed
-
- Harris RC. The macula densa: recent developments. J Hypertens. 1996;14:815–822. - PubMed
-
- Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–R279. - PubMed
-
- Kotchen TA, Galla JH, Luke RG. Failure of NaHCO3 and KHCO3 to inhibit renin in the rat. Am J Physiol. 1976;231:1050–1056. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

