Impaired growth and elevated fas receptor expression in PIGA(+) stem cells in primary paroxysmal nocturnal hemoglobinuria
- PMID: 10974022
- PMCID: PMC381282
- DOI: 10.1172/JCI8328
Impaired growth and elevated fas receptor expression in PIGA(+) stem cells in primary paroxysmal nocturnal hemoglobinuria
Abstract
The genetic defect underlying paroxysmal nocturnal hemoglobinuria (PNH) has been shown to reside in PIGA, a gene that encodes an element required for the first step in glycophosphatidylinositol anchor assembly. Why PIGA-mutated cells are able to expand in PNH marrow, however, is as yet unclear. To address this question, we compared the growth of affected CD59(-)CD34(+) and unaffected CD59(+)CD34(+) cells from patients with that of normal CD59(+)CD34(+) cells in liquid culture. One hundred FACS-sorted cells were added per well into microtiter plates, and after 11 days at 37 degrees C the progeny were counted and were analyzed for their differentiation pattern. We found that CD59(-)CD34(+) cells from PNH patients proliferated to levels approaching those of normal cells, but that CD59(+)CD34(+) cells from the patients gave rise to 20- to 140-fold fewer cells. Prior to sorting, the patients' CD59(-) and CD59(+)CD34(+) cells were equivalent with respect to early differentiation markers, and following culture, the CD45 differentiation patterns were identical to those of control CD34(+) cells. Further analyses of the unsorted CD59(+)CD34(+) population, however, showed elevated levels of Fas receptor. Addition of agonist anti-Fas mAb to cultures reduced the CD59(+)CD34(+) cell yield by up to 78% but had a minimal effect on the CD59(-)CD34(+) cells, whereas antagonist anti-Fas mAb enhanced the yield by up to 250%. These results suggest that expansion of PIGA-mutated cells in PNH marrow is due to a growth defect in nonmutated cells, and that greater susceptibility to apoptosis is one factor involved in the growth impairment.
Figures
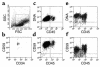
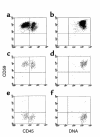



Comment in
-
Genetic and environmental effects in paroxysmal nocturnal hemoglobinuria: this little PIG-A goes "Why? Why? Why?".J Clin Invest. 2000 Sep;106(5):637-41. doi: 10.1172/JCI11002. J Clin Invest. 2000. PMID: 10974016 Free PMC article. No abstract available.
References
-
- Yomtovian R, Prince GM, Medof ME. The molecular basis for paroxysmal nocturnal hemoglobinuria. Transfusion. 1993;33:852–873. - PubMed
-
- Armstrong C, et al. Affected paroxysmal nocturnal hemoglobinuria T lymphocytes harbor a common defect in assembly of N-acetyl-D-glucosamine inositol phospholipid corresponding to that in class A Thy-1- murine lymphoma mutants. J Biol Chem. 1992;267:25347–25351. - PubMed
-
- Takeda J, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73:703–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

