B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells
- PMID: 10974030
- PMCID: PMC2193277
- DOI: 10.1084/jem.192.5.637
B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells
Abstract
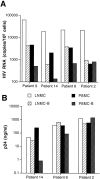
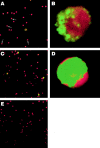
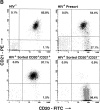
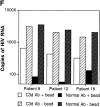
The impact of HIV-associated immunopathogenesis on B cells has been largely associated with indirect consequences of viral replication. This study demonstrates that HIV interacts directly with B cells in both lymphoid tissues and peripheral blood. B cells isolated from lymph node and peripheral blood mononuclear cells (PBMCs) of 4 and 23 chronically infected patients, respectively, demonstrated similar capacities to pass virus to activated HIV-negative PBMCs when compared with CD4(+) cells from the same patients. However, in contrast to T cells, virus associated with B cells was surface bound, as shown by its sensitivity to pronase and the staining pattern revealed by in situ amplification of HIV-1 RNA. Cell sorting and ligand displacing approaches established that CD21 was the HIV-binding receptor on B cells, and that this association was mediated through complement-opsonized virus. These B cells were also found to express significantly lower levels of CD21 compared with HIV-negative individuals, suggesting a direct perturbing effect of HIV on B cells. These findings suggest that B cells, although they themselves are not readily infected by HIV, are similar to follicular dendritic cells in their capacity to serve as extracellular reservoirs for HIV-1. Furthermore, B cells possess the added capability of circulating in peripheral blood and migrating through tissues where they can potentially interact with and pass virus to T cells.
Figures
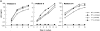
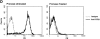
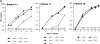
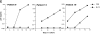
References
-
- Burton G.F., Masuda A., Heath S.L., Smith B.A., Tew J.G., Szakal A.K. Follicular dendritic cells (FDC) in retroviral infectionhost/pathogen perspectives. Immunol. Rev. 1997;156:185–197. - PubMed
-
- Haase A.T. Population biology of HIV-1 infectionviral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu. Rev. Immunol. 1999;17:625–656. - PubMed
-
- Geijtenbeek T.B., Kwon D.S., Torensma R., van Vliet S.J., van Duijnhoven G.C., Middel J., Cornelissen I.L., Nottet H.S., KewalRamani V.N., Littman D.R. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. - PubMed
-
- Liao Z., Roos J.W., Hildreth J.E. Increased infectivity of HIV type 1 particles bound to cell surface and solid-phase ICAM-1 and VCAM-1 through acquired adhesion molecules LFA-1 and VLA-4. AIDS Res. Hum. Retroviruses. 2000;16:355–366. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

