Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor
- PMID: 10974032
- PMCID: PMC2193276
- DOI: 10.1084/jem.192.5.659
Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor
Abstract
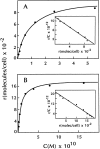
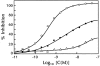
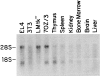
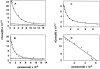
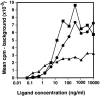
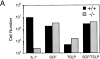
The cellular receptor for murine thymic stromal lymphopoietin (TSLP) was detected in a variety of murine, but not human myelomonocytic cell lines by radioligand binding. cDNA clones encoding the receptor were isolated from a murine T helper cell cDNA library. TSLP receptor (TSLPR) is a member of the hematopoietin receptor family. Transfection of TSLPR cDNA resulted in only low affinity binding. Cotransfection of the interleukin 7 (IL-7)Ralpha chain cDNA resulted in conversion to high affinity binding. TSLP did not activate cells from IL-7Ralpha(-/)- mice, but did activate cells from gammac(-/)- mice. Thus, the functional TSLPR requires the IL-7Ralpha chain, but not the gammac chain for signaling.
Figures

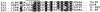


References
-
- Komschlies K.L., Grzegorzewski K.J., Wiltrout R.H. Diverse immunological and hematological effects of interleukin-7implications for clinical application. J. Leukoc. Biol. 1995;58:623–633. - PubMed
-
- Di Santo J.P., Kühn R., Müller W. Common cytokine receptor γ chain (γc)-dependent cytokinesunderstanding in vivo functions by gene targeting. Immunol. Rev. 1995;148:19–34. - PubMed
-
- Ihle J.N., Witthuhn B.A., Quelle F.W., Yamamoto K., Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu. Rev. Immunol. 1995;13:369–398. - PubMed
-
- Chomarat P., Banchereau J. An update on Interleukin-4 and its receptor. Eur. Cytokine Netw. 1997;8:333–344. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

