Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma
- PMID: 10974040
- PMCID: PMC2193269
- DOI: 10.1084/jem.192.5.755
Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma
Abstract
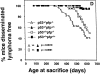
Immune surveillance by cytotoxic lymphocytes against cancer has been postulated for decades, but direct evidence for the role of cytotoxic lymphocytes in protecting against spontaneous malignancy has been lacking. As the rejection of many experimental cancers by cytotoxic T lymphocytes and natural killer cells is dependent on the pore-forming protein perforin (pfp), we examined pfp-deficient mice for increased cancer susceptibility. Here we show that pfp-deficient mice have a high incidence of malignancy in distinct lymphoid cell lineages (T, B, NKT), indicating a specific requirement for pfp in protection against lymphomagenesis. The susceptibility to lymphoma was accentuated by simultaneous lack of expression of the p53 gene, mutations in which also commonly predispose to human malignancies, including lymphoma. In contrast, the incidence and age of onset of sarcoma was unaffected in p53-deficient mice. Pfp-deficient mice were at least 1,000-fold more susceptible to these lymphomas when transplanted, compared with immunocompetent mice in which tumor rejection was controlled by CD8(+) T lymphocytes. This study is the first that implicates direct cytotoxicity by lymphocytes in regulating lymphomagenesis.
Figures




References
-
- Burnet F.M. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. - PubMed
-
- Melief C.J., Kast W.M. Cytotoxic T lymphocyte therapy of cancer and tumor escape mechanisms. Semin. Cancer Biol. 1991;2:347–354. - PubMed
-
- Whiteside T.L., Vujanovic N.L., Herberman R.B. Natural killer cells and tumor therapy. Curr. Top. Microbiol. Immunol. 1998;230:221–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

