Lytic cycle of Toxoplasma gondii
- PMID: 10974128
- PMCID: PMC99006
- DOI: 10.1128/MMBR.64.3.607-623.2000
Lytic cycle of Toxoplasma gondii
Abstract
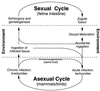
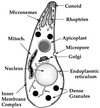
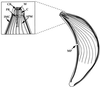
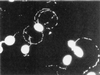
Toxoplasma gondii is an obligate intracellular pathogen within the phylum Apicomplexa. This protozoan parasite is one of the most widespread, with a broad host range including many birds and mammals and a geographic range that is nearly worldwide. While infection of healthy adults is usually relatively mild, serious disease can result in utero or when the host is immunocompromised. This sophisticated eukaryote has many specialized features that make it well suited to its intracellular lifestyle. In this review, we describe the current knowledge of how the asexual tachyzoite stage of Toxoplasma attaches to, invades, replicates in, and exits the host cell. Since this process is closely analogous to the way in which viruses reproduce, we refer to it as the Toxoplasma "lytic cycle."
Figures
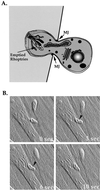

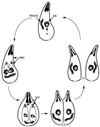

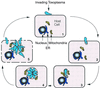
References
-
- Achbarou A, Mercereau P, Sadak A, Gortier B, Leriche M A, Camus D, Dubremetz J F. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology. 1991;3:321–329. - PubMed
-
- Achbarou A, Mercereau-Puijalon O, Autheman J M, Fortier B, Camus D, Dubremetz J F. Characterization of microneme proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1991;47:223–233. - PubMed
-
- Ajioka J W, Boothroyd J C, Brunk B P, Hehl A, Hillier L, Manger I D, Marra M, Overton G C, Roos D S, Wan K L, Waterston R, Sibley L D. Gene discovery by EST sequencing in Toxoplasma gondii reveals sequences restricted to the Apicomplexa. Genome Res. 1998;8:18–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

