Synthesis of a bacteriophage MB78 late protein by novel ribosomal frameshifting
- PMID: 10974552
- PMCID: PMC7173172
- DOI: 10.1016/s0378-1119(00)00264-x
Synthesis of a bacteriophage MB78 late protein by novel ribosomal frameshifting
Abstract
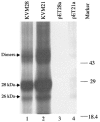
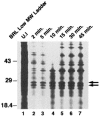
MB78 is a virulent phage of Salmonella typhimurium that possesses a number of interesting features, making it a suitable organism to study the regulation of gene expression. A detailed physical map of this phage genome has been constructed and is being extensively studied at the molecular level. Here, we demonstrate the expression of two late proteins of bacteriophage MB78 derived from the same gene as a result of possible ribosomal frameshifting. In vitro transcription-translation yields a major protein that migrates as 28kDa, whereas in vivo expression using pET expression vectors yields two equally expressed proteins of molecular sizes 28 and 26kDa. A putative slippery sequence TTTAAAG and a pseudoknot structure, two essential cis elements required for the classical ribosomal frameshifting, are identified in the reading frame. Mutations created at the slippery sequence resulted in a single 28kDa protein and completely abolished the expression of 26kDa protein. Thus, we have produced the first evidence that ribosomal frameshifting occurs in bacteriophage MB78 of Salmonella typhimurium.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

