In situ localization of C3 synthesis in experimental acute renal allograft rejection
- PMID: 10980122
- PMCID: PMC1885894
- DOI: 10.1016/S0002-9440(10)64596-8
In situ localization of C3 synthesis in experimental acute renal allograft rejection
Abstract
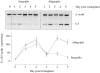
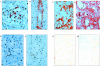
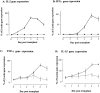
Recent evidence has implicated complement in renal transplant injury and identified the kidney as a source of complement components. We therefore investigated the local gene expression of complement component C3, pivotal to complement activation pathways and a mediator of inflammatory injury, in a rat renal transplant model. By reverse transcriptase-polymerase chain reaction, the expression of C3 mRNA increased in two phases. The first phase coincided with post-ischemic injury over 2 days post-transplantation and was localized by in situ hybridization to vessels and glomerular mesangial cells in allogeneic and syngeneic (control) kidney transplants. In allografts only, a second phase was found in tubular epithelial cells, glomerular parietal cells, vessel walls and some infiltrating cells, which peaked on day 4 together with rapid influx of leukocytes, tubule cell damage, the induction of interleukin-2 and interferon-gamma mRNA, and the up-regulation of tumor necrosis factor-alpha and interleukin-1beta mRNA in the graft. In vitro studies showed that interleukin-2 and interferon-gamma up-regulate C3 production in renal tubule cells. We conclude that post-ischemic injury led to transient up-regulation of glomerular expression of C3 mRNA. Subsequent cellular rejection was associated with tubulointerstitial/glomerular parietal cell expression of C3 mRNA. This differential expression of local C3, immediately post-transplant or associated with acute rejection, may have implications for putative therapeutic complement inhibition in clinical transplantation.
Figures


References
-
- Erdei A, Fust G, Gergely J: The role of C3 in the immune response. Immunol Today 1991, 12:332-337 - PubMed
-
- Mathew M, Bolton WK: Linear C3 deposits on the tubular basement membrane in renal allograft biopsies. Am J Kidney Dis 1988, 12:121-125 - PubMed
-
- Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Reithmuller G, Land W, Albert E: Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 1993, 43:1333-1338 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

