Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence
- PMID: 10980131
- PMCID: PMC1885689
- DOI: 10.1016/S0002-9440(10)64605-6
Expression of p57(KIP2) potently blocks the growth of human astrocytomas and induces cell senescence
Abstract
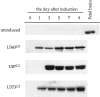
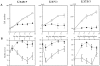
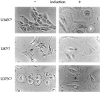
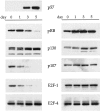
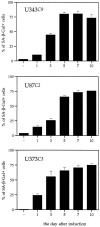
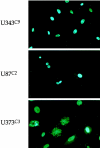
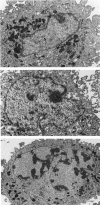
Astrocytic tumors frequently exhibit defects in the expression or activity of proteins that control cell-cycle progression. Inhibition of kinase activity associated with cyclin/cyclin-dependent kinase co-complexes by cyclin-dependent kinase inhibitors is an important mechanism by which the effects of growth signals are down-regulated. We undertook the present study to determine the role of p57(KIP2) (p57) in human astrocytomas. We demonstrate here that whereas p57 is expressed in fetal brain tissue, specimens of astrocytomas of varying grade and permanent astrocytoma cell lines do not express p57, and do not contain mutations of the p57 gene by multiplex-heteroduplex analysis. However, the inducible expression of p57 in three well-characterized human astrocytoma cell lines (U343 MG-A, U87 MG, and U373 MG) using the tetracycline repressor system leads to a potent proliferative block in G(1) as determined by growth curve and flow cytometric analyses. After the induction of p57, retinoblastoma protein, p107, and E2F-1 levels diminish, and retinoblastoma protein is shifted to a hypophosphorylated form. Morphologically, p57-induced astrocytoma cells became large and flat with an expanded cytoplasm. The inducible expression of p57 leads to the accumulation of senescence-associated beta-galactosidase marker within all astrocytoma cell lines such that approximately 75% of cells were positive at 1 week after induction. Induction of p57 in U373 astrocytoma cells generated a small population of cells ( approximately 15%) that were nonviable, contained discrete nuclear fragments on Hoechst 33258 staining, and demonstrated ultrastructural features characteristic of apoptosis. Examination of bax and poly-(ADP ribose) polymerase levels showed no change in bax, but decreased expression of poly-(ADP ribose) polymerase after p57 induction in all astrocytoma cell lines. These data demonstrate that the proliferative block imposed by p57 on human astrocytoma cells results in changes in the expression of a number of cell cycle regulatory factors, cell morphology, and a strong stimulus to cell senescence.
Figures





Similar articles
-
The E2F-family proteins induce distinct cell cycle regulatory factors in p16-arrested, U343 astrocytoma cells.Oncogene. 1998 Aug 20;17(7):867-76. doi: 10.1038/sj.onc.1202008. Oncogene. 1998. PMID: 9780003
-
Activation of a cAMP pathway and induction of melanogenesis correlate with association of p16(INK4) and p27(KIP1) to CDKs, loss of E2F-binding activity, and premature senescence of human melanocytes.Exp Cell Res. 1999 Dec 15;253(2):561-72. doi: 10.1006/excr.1999.4688. Exp Cell Res. 1999. PMID: 10585280
-
Expression and activity of the retinoblastoma protein (pRB)-family proteins, p107 and p130, during L6 myoblast differentiation.Cell Growth Differ. 1995 Oct;6(10):1287-98. Cell Growth Differ. 1995. PMID: 8845306
-
Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation.Crit Rev Biochem Mol Biol. 1996 Jun;31(3):237-71. doi: 10.3109/10409239609106585. Crit Rev Biochem Mol Biol. 1996. PMID: 8817077 Review.
-
Growth suppression by members of the retinoblastoma protein family.Cold Spring Harb Symp Quant Biol. 1994;59:75-84. doi: 10.1101/sqb.1994.059.01.011. Cold Spring Harb Symp Quant Biol. 1994. PMID: 7587134 Review. No abstract available.
Cited by
-
Accelerated aging-related transcriptome alterations in neurovascular unit cells in the brain of Alzheimer's disease.Front Aging Neurosci. 2022 Aug 18;14:949074. doi: 10.3389/fnagi.2022.949074. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36062157 Free PMC article.
-
Therapy-induced senescence in cancer.J Natl Cancer Inst. 2010 Oct 20;102(20):1536-46. doi: 10.1093/jnci/djq364. Epub 2010 Sep 21. J Natl Cancer Inst. 2010. PMID: 20858887 Free PMC article. Review.
-
Genome amplification and cellular senescence are hallmarks of human placenta development.PLoS Genet. 2018 Oct 12;14(10):e1007698. doi: 10.1371/journal.pgen.1007698. eCollection 2018 Oct. PLoS Genet. 2018. PMID: 30312291 Free PMC article.
-
ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases.Oxid Med Cell Longev. 2016;2016:3565127. doi: 10.1155/2016/3565127. Epub 2016 May 10. Oxid Med Cell Longev. 2016. PMID: 27247702 Free PMC article. Review.
-
Genetic syndromes associated with overgrowth in childhood.Ann Pediatr Endocrinol Metab. 2013 Sep;18(3):101-5. doi: 10.6065/apem.2013.18.3.101. Epub 2013 Sep 30. Ann Pediatr Endocrinol Metab. 2013. PMID: 24904861 Free PMC article. Review.
References
-
- Dirks PB, Murakami M, Hubbard SL, Rutka JT: Cyclins and cyclin-dependent kinase expression in human astrocytoma cell lines. J Neuropathol Exp Neurol 1997, 56:291-300 - PubMed
-
- Dirks PB, Rutka JT: Current concepts in neuro-oncology. The cell cycle—a review. Neurosurgery 1997, 40:1000-1015 - PubMed
-
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogetstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 - PubMed
-
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D: p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366:701-704 - PubMed
-
- Polyak K, Lee M-H, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagne J: Cloning of p21Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 8:59-66 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

