A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis
- PMID: 10982364
- PMCID: PMC102116
- DOI: 10.1128/jvi.74.19.9175-9183.2000
A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis
Abstract
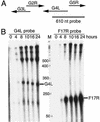
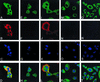
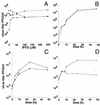
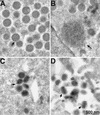
Vaccinia virus encodes two glutaredoxins, O2L and G4L, both of which exhibit thioltransferase and dehydroascorbate reductase activities in vitro. Although O2L was previously found to be dispensable for virus replication, we now show that G4L is necessary for virion morphogenesis. RNase protection and Western blotting assays indicated that G4L was expressed at late times after infection and was incorporated into mature virus particles. Attempts to isolate a mutant virus with a deleted G4L gene were unsuccessful, suggesting that the protein was required for virus replication. This interpretation was confirmed by the construction and characterization of a conditional lethal recombinant virus with an inducible copy of the G4L gene replacing the original one. Expression of G4L was proportional to the concentration of inducer, and the amount of glutaredoxin could be varied from barely detectable to greater than normal amounts of protein. Immunogold labeling revealed that the induced G4L protein was associated with immature and mature virions and adjacent cytoplasmic depots. In the absence of inducer, the production of infectious virus was severely inhibited, though viral late protein synthesis appeared unaffected except for decreased maturation-dependent proteolytic processing of certain core components. Electron microscopy of cells infected under nonpermissive conditions revealed an accumulation of crescent membranes on the periphery of electron-dense globular masses but few mature particles. We concluded that the two glutaredoxin homologs encoded by vaccinia virus have different functions and that G4L has a role in virion morphogenesis, perhaps by acting as a redox protein.
Figures







Similar articles
-
Vaccinia virus G4L gene encodes a second glutaredoxin.Virology. 1996 Dec 15;226(2):408-11. doi: 10.1006/viro.1996.0669. Virology. 1996. PMID: 8955061
-
Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly.J Virol. 2002 Jan;76(2):467-72. doi: 10.1128/jvi.76.2.467-472.2002. J Virol. 2002. PMID: 11752136 Free PMC article.
-
Vaccinia virus A30L protein is required for association of viral membranes with dense viroplasm to form immature virions.J Virol. 2001 Jul;75(13):5752-61. doi: 10.1128/JVI.75.13.5752-5761.2001. J Virol. 2001. PMID: 11390577 Free PMC article.
-
Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascorbate reductase activities.Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7060-4. doi: 10.1073/pnas.89.15.7060. Proc Natl Acad Sci U S A. 1992. PMID: 1496000 Free PMC article.
-
From crescent to mature virion: vaccinia virus assembly and maturation.Viruses. 2014 Oct 7;6(10):3787-808. doi: 10.3390/v6103787. Viruses. 2014. PMID: 25296112 Free PMC article. Review.
Cited by
-
The Viral Protein Poly(A) Polymerase Catalytic Subunit Interacts with Guanylate-Binding Proteins 2 to Antagonize the Antiviral Ability of Targeting Ectromelia Virus.Int J Mol Sci. 2023 Oct 30;24(21):15750. doi: 10.3390/ijms242115750. Int J Mol Sci. 2023. PMID: 37958732 Free PMC article.
-
Analysis of the monkeypox virus genome.Virology. 2002 Jun 5;297(2):172-94. doi: 10.1006/viro.2002.1446. Virology. 2002. PMID: 12083817 Free PMC article.
-
Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling.Antioxid Redox Signal. 2013 Nov 1;19(13):1539-605. doi: 10.1089/ars.2012.4599. Epub 2013 Mar 28. Antioxid Redox Signal. 2013. PMID: 23397885 Free PMC article. Review.
-
The myristate moiety and amino terminus of vaccinia virus l1 constitute a bipartite functional region needed for entry.J Virol. 2012 May;86(10):5437-51. doi: 10.1128/JVI.06703-11. Epub 2012 Mar 7. J Virol. 2012. PMID: 22398293 Free PMC article.
-
African swine fever virus pB119L protein is a flavin adenine dinucleotide-linked sulfhydryl oxidase.J Virol. 2006 Apr;80(7):3157-66. doi: 10.1128/JVI.80.7.3157-3166.2006. J Virol. 2006. PMID: 16537584 Free PMC article.
References
-
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

