Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation
- PMID: 10982365
- PMCID: PMC102117
- DOI: 10.1128/jvi.74.19.9184-9196.2000
Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation
Abstract
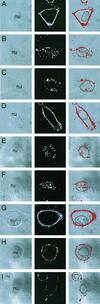
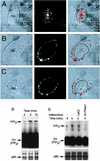
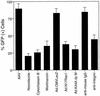
Adeno-associated virus (AAV) is a single-stranded DNA parvovirus that causes no currently known pathology in humans. Despite the fact that this virus is of increasing interest to molecular medicine as a vector for gene delivery, relatively little is known about the cellular mechanisms controlling infection. In this study, we have examined endocytic and intracellular trafficking of AAV-2 using fluorescent (Cy3)-conjugated viral particles and molecular techniques. Our results demonstrate that internalization of heparan sulfate proteoglycan-bound AAV-2 requires alphaVbeta5 integrin and activation of the small GTP-binding protein Rac1. Following endocytosis, activation of a phosphatidylinositol-3 (PI3) kinase pathway was necessary to initiate intracellular movement of AAV-2 to the nucleus via both microfilaments and microtubules. Inhibition of Rac1 using a dominant N17Rac1 mutant led to a decrease in AAV-2-mediated PI3 kinase activation, indicating that Rac1 may act proximal to PI3 kinase during AAV-2 infection. In summary, our results indicate that alphaVbeta5 integrin-mediated endocytosis of AAV-2 occurs through a Rac1 and PI3 kinase activation cascade, which directs viral movement along the cytoskeletal network to the nucleus.
Figures






References
-
- Ali R R, Reichel M B, Thrasher A J, Levinsky R J, Kinnon C, Kanuga N, Hunt D M, Bhattacharya S S. Gene transfer into the mouse retina mediated by an adeno-associated viral vector. Hum Mol Genet. 1996;5:591–594. - PubMed
-
- Bagrodia S, Taylor S J, Jordon K A, Van Aelst L, Cerione R A. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. - PubMed
-
- Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

