The unique catalytic subunit of sperm cAMP-dependent protein kinase is the product of an alternative Calpha mRNA expressed specifically in spermatogenic cells
- PMID: 10982398
- PMCID: PMC14973
- DOI: 10.1091/mbc.11.9.3031
The unique catalytic subunit of sperm cAMP-dependent protein kinase is the product of an alternative Calpha mRNA expressed specifically in spermatogenic cells
Abstract
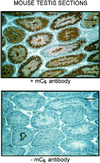
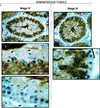
cAMP-dependent protein kinase has a central role in the control of mammalian sperm capacitation and motility. Previous protein biochemical studies indicated that the only cAMP-dependent protein kinase catalytic subunit (C) in ovine sperm is an unusual isoform, termed C(s), whose amino terminus differs from those of published C isoforms of other species. Isolation and sequencing of cDNA clones encoding ovine C(s) and Calpha1 (the predominant somatic isoform) now reveal that C(s) is the product of an alternative transcript of the Calpha gene. C(s) cDNA clones from murine and human testes also were isolated and sequenced, indicating that C(s) is of ancient origin and widespread in mammals. In the mouse, C(s) transcripts were detected only in testis and not in any other tissue examined, including ciliated tissues and ovaries. Finally, immunohistochemistry of the testis shows that C(s) first appears in pachytene spermatocytes. This is the first demonstration of a cell type-specific expression for any C isoform. The conservation of C(s) throughout mammalian evolution suggests that the unique structure of C(s) is important in the subunit's localization or function within the sperm.
Figures







References
-
- Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterized by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111:645–656. - PubMed
-
- Amat JA, Fields KL, Schubart UK. Stage-specific expression of phosphoprotein p19 during spermatogenesis in the rat. Mol Reprod Dev. 1990;26:383–390. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Protocols in Molecular Biology. Vol. 1. New York: John Wiley & Sons; 1989.
-
- Beebe SJ, Øyen O, Sandberg M, Frøysa A, Hansson V, Jahnsen T. Molecular cloning of a tissue-specific protein kinase (Cγ) from human testis: representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990;4:465–475. - PubMed
-
- Brokaw CJ. Regulation of sperm flagellar motility by calcium and cAMP-dependent phosphorylation. J Cell Biochem. 1987;35:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

