Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization
- PMID: 10982405
- PMCID: PMC14980
- DOI: 10.1091/mbc.11.9.3123
Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization
Abstract
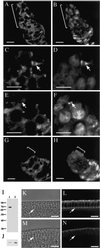
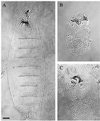
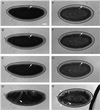
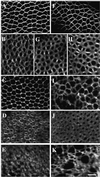
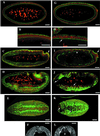
The septins are a conserved family of proteins that are involved in cytokinesis and other aspects of cell-surface organization. In Drosophila melanogaster, null mutations in the pnut septin gene are recessive lethal, but homozygous pnut mutants complete embryogenesis and survive until the pupal stage. Because the completion of cellularization and other aspects of early development seemed likely to be due to maternally contributed Pnut product, we attempted to generate embryos lacking the maternal contribution in order to explore the roles of Pnut in these processes. We used two methods, the production of germline clones homozygous for a pnut mutation and the rescue of pnut homozygous mutant flies by a pnut(+) transgene under control of the hsp70 promoter. Remarkably, the pnut germline-clone females produced eggs, indicating that stem-cell and cystoblast divisions in the female germline do not require Pnut. Moreover, the Pnut-deficient embryos obtained by either method completed early syncytial development and began cellularization of the embryo normally. However, during the later stages of cellularization, the organization of the actin cytoskeleton at the leading edge of the invaginating furrows became progressively more abnormal, and the embryos displayed widespread defects in cell and embryo morphology beginning at gastrulation. Examination of two other septins showed that Sep1 was not detectable at the cellularization front in the Pnut-deficient embryos, whereas Sep2 was still present in normal levels. Thus, it is possible that Sep2 (perhaps in conjunction with other septins such as Sep4 and Sep5) fulfills an essential septin role during the organization and initial ingression of the cellularization furrow even in the absence of Pnut and Sep1. Together, the results suggest that some cell-division events in Drosophila do not require septin function, that there is functional differentiation among the Drosophila septins, or both.
Figures

References
-
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2:434–439. - PubMed
-
- Caltagarone J, Rhodes J, Honer WG, Bowser R. Localization of a novel septin protein, hCDCrel-1, in neurons of human brain. NeuroReport. 1998;9:2907–2912. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

