Prion-dependent switching between respiratory competence and deficiency in the yeast nam9-1 mutant
- PMID: 10982839
- PMCID: PMC86276
- DOI: 10.1128/MCB.20.19.7220-7229.2000
Prion-dependent switching between respiratory competence and deficiency in the yeast nam9-1 mutant
Abstract
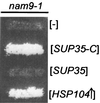
Nam9p is a protein of the mitochondrial ribosome. The respiration-deficient Saccharomyces cerevisiae strain MB43-nam9-1 expresses Nam9-1p containing the point mutation S82L. Respiratory deficiency correlates with a decrease in the steady level of some mitochondrially encoded proteins and the complete lack of mitochondrially encoded cytochrome oxidase subunit 2 (Cox2). De novo synthesis of Cox2 in MB43-nam9-1 is unaffected, indicating that newly synthesized Cox2 is rapidly degraded. Respiratory deficiency of MB43-nam9-1 is overcome by transient overexpression of HSP104, by deletion of HSP104, by transient exposure to guanidine hydrochloride, and by expression of the C-terminal portion of Sup35, indicating an involvement of the yeast prion [PSI(+)]. Respiratory deficiency of MB43-nam9-1 can be reinduced by transfer of cytosol from S. cerevisiae that harbors [PSI(+)]. We conclude that nam9-1 causes respiratory deficiency only in combination with the cytosolic prion [PSI(+)], presenting the first example of a synthetic effect between cytosolic [PSI(+)] and a mutant mitochondrial protein.
Figures

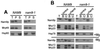




Similar articles
-
The [PSI+] prion modulates cytochrome c oxidase deficiency caused by deletion of COX12.Mol Biol Cell. 2022 Dec 1;33(14):ar130. doi: 10.1091/mbc.E21-10-0499. Epub 2022 Sep 21. Mol Biol Cell. 2022. PMID: 36129767 Free PMC article.
-
NAM9 nuclear suppressor of mitochondrial ochre mutations in Saccharomyces cerevisiae codes for a protein homologous to S4 ribosomal proteins from chloroplasts, bacteria, and eucaryotes.Mol Cell Biol. 1992 Jan;12(1):402-12. doi: 10.1128/mcb.12.1.402-412.1992. Mol Cell Biol. 1992. PMID: 1729612 Free PMC article.
-
Rpm2, the protein subunit of mitochondrial RNase P in Saccharomyces cerevisiae, also has a role in the translation of mitochondrially encoded subunits of cytochrome c oxidase.Genetics. 2001 Jun;158(2):573-85. doi: 10.1093/genetics/158.2.573. Genetics. 2001. PMID: 11404323 Free PMC article.
-
Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae.Genetics. 1997 Oct;147(2):507-19. doi: 10.1093/genetics/147.2.507. Genetics. 1997. PMID: 9335589 Free PMC article.
-
Protein-only inheritance in yeast: something to get [PSI+]-ched about.Trends Cell Biol. 2000 Mar;10(3):98-105. doi: 10.1016/s0962-8924(99)01711-0. Trends Cell Biol. 2000. PMID: 10675903 Review.
Cited by
-
A probabilistic framework for peptide and protein quantification from data-dependent and data-independent LC-MS proteomics experiments.OMICS. 2012 Sep;16(9):468-82. doi: 10.1089/omi.2012.0019. Epub 2012 Aug 7. OMICS. 2012. PMID: 22871168 Free PMC article.
-
The [PSI+] prion modulates cytochrome c oxidase deficiency caused by deletion of COX12.Mol Biol Cell. 2022 Dec 1;33(14):ar130. doi: 10.1091/mbc.E21-10-0499. Epub 2022 Sep 21. Mol Biol Cell. 2022. PMID: 36129767 Free PMC article.
-
Cytosolic aggregation of mitochondrial proteins disrupts cellular homeostasis by stimulating the aggregation of other proteins.Elife. 2021 Jul 20;10:e65484. doi: 10.7554/eLife.65484. Elife. 2021. PMID: 34292154 Free PMC article.
-
Screening for toxic amyloid in yeast exemplifies the role of alternative pathway responsible for cytotoxicity.PLoS One. 2009;4(3):e4539. doi: 10.1371/journal.pone.0004539. Epub 2009 Mar 5. PLoS One. 2009. PMID: 19262694 Free PMC article.
-
The ribosome-bound chaperones RAC and Ssb1/2p are required for accurate translation in Saccharomyces cerevisiae.Mol Cell Biol. 2004 Oct;24(20):9186-97. doi: 10.1128/MCB.24.20.9186-9197.2004. Mol Cell Biol. 2004. PMID: 15456889 Free PMC article.
References
-
- Altamura N, Dujardin G, Groudinsky O, Slonimski P P. Two adjacent nuclear genes, ISF1 and NAM7/UPF1, cooperatively participate in mitochondrial functions in Saccharomyces cerevisiae. Mol Gen Genet. 1994;242:49–56. - PubMed
-
- Biswas T K, Getz G S. The single amino acid changes in the yeast mitochondrial S4 ribosomal protein cause temperature-sensitive defect in the accumulation of mitochondrial 15S rRNA. Biochemistry. 1999;38:13042–13054. - PubMed
-
- Boguta M, Chacinska A, Murawski M, Szczesniak B. Expression of the yeast NAM9 gene coding for mitochondrial ribosomal protein. Acta Biochim Pol. 1997;44:251–258. - PubMed
-
- Boguta M, Dmochowksa A, Borsuk P, Wrobel K, Gargouri A, Lazowska J, Slonimski P P, Szczesniak B, Kruszewska A. NAM9 nuclear suppressor of mitochondrial ochre mutations in Saccharomyces cerevisiae codes for a protein homologous to S4 ribosomal proteins from chloroplasts, bacteria, and eucaryotes. Mol Cell Biol. 1992;12:402–412. - PMC - PubMed
-
- Bonnefoy N, Chalvet F, Hamel P, Slonimski P P, Dujardin G. OXA1, a Saccharomyces cerevisiae nuclear gene whose sequence is conserved from prokaryotes to eukaryotes controls cytochrome oxidase biogenesis. J Mol Biol. 1994;239:201–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
