Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells
- PMID: 10982854
- PMCID: PMC86291
- DOI: 10.1128/MCB.20.19.7378-7387.2000
Small GTPase RhoG is a key regulator for neurite outgrowth in PC12 cells
Abstract
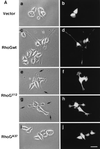
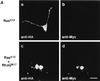
The Rho family of small GTPases has been implicated in cytoskeletal reorganization and subsequent morphological changes in various cell types. Among them, Rac and Cdc42 have been shown to be involved in neurite outgrowth in neuronal cells. In this study, we examined the role of RhoG, another member of Rho family GTPases, in nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells. Expression of wild-type RhoG in PC12 cells induced neurite outgrowth in the absence of NGF, and the morphology of wild-type RhoG-expressing cells was similar to that of NGF-differentiated cells. Constitutively active RhoG-transfected cells extended short neurites but developed large lamellipodial or filopodial structures at the tips of neurites. RhoG-induced neurite outgrowth was inhibited by coexpression with dominant-negative Rac1 or Cdc42. In addition, expression of constitutively active RhoG elevated endogenous Rac1 and Cdc42 activities. We also found that the NGF-induced neurite outgrowth was enhanced by expression of wild-type RhoG whereas expression of dominant-negative RhoG suppressed the neurite outgrowth. Furthermore, constitutively active Ras-induced neurite outgrowth was also suppressed by dominant-negative RhoG. Taken together, these results suggest that RhoG is a key regulator in NGF-induced neurite outgrowth, acting downstream of Ras and upstream of Rac1 and Cdc42 in PC12 cells.
Figures









Similar articles
-
The Human Rho-GEF trio and its target GTPase RhoG are involved in the NGF pathway, leading to neurite outgrowth.Curr Biol. 2002 Feb 19;12(4):307-12. doi: 10.1016/s0960-9822(02)00658-9. Curr Biol. 2002. PMID: 11864571
-
The c-Fes tyrosine kinase cooperates with the breakpoint cluster region protein (Bcr) to induce neurite extension in a Rac- and Cdc42-dependent manner.Exp Cell Res. 2004 Sep 10;299(1):188-98. doi: 10.1016/j.yexcr.2004.05.010. Exp Cell Res. 2004. PMID: 15302586
-
Spatio-temporal regulation of Rac1 and Cdc42 activity during nerve growth factor-induced neurite outgrowth in PC12 cells.J Biol Chem. 2004 Jan 2;279(1):713-9. doi: 10.1074/jbc.M306382200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570905
-
In PC12 cells, expression of neurosecretion and neurite outgrowth are governed by the transcription repressor REST/NRSF.Cell Mol Neurobiol. 2010 Nov;30(8):1295-302. doi: 10.1007/s10571-010-9602-0. Epub 2010 Nov 3. Cell Mol Neurobiol. 2010. PMID: 21046448 Free PMC article. Review.
-
Regulation of neurite outgrowth by G(i/o) signaling pathways.Front Biosci. 2008 May 1;13:4544-57. doi: 10.2741/3022. Front Biosci. 2008. PMID: 18508528 Free PMC article. Review.
Cited by
-
miR-124-regulated RhoG reduces neuronal process complexity via ELMO/Dock180/Rac1 and Cdc42 signalling.EMBO J. 2012 Jun 29;31(13):2908-21. doi: 10.1038/emboj.2012.130. Epub 2012 May 15. EMBO J. 2012. PMID: 22588079 Free PMC article.
-
The small GTPase RhoG mediates glioblastoma cell invasion.Mol Cancer. 2012 Sep 11;11:65. doi: 10.1186/1476-4598-11-65. Mol Cancer. 2012. PMID: 22966858 Free PMC article.
-
A role of Rnd1 GTPase in dendritic spine formation in hippocampal neurons.J Neurosci. 2003 Dec 3;23(35):11065-72. doi: 10.1523/JNEUROSCI.23-35-11065.2003. J Neurosci. 2003. PMID: 14657163 Free PMC article.
-
The Arf family GTPase Arl4A complexes with ELMO proteins to promote actin cytoskeleton remodeling and reveals a versatile Ras-binding domain in the ELMO proteins family.J Biol Chem. 2011 Nov 11;286(45):38969-79. doi: 10.1074/jbc.M111.274191. Epub 2011 Sep 19. J Biol Chem. 2011. PMID: 21930703 Free PMC article.
-
Apoptotic cell recognition receptors and scavenger receptors.Immunol Rev. 2016 Jan;269(1):44-59. doi: 10.1111/imr.12376. Immunol Rev. 2016. PMID: 26683144 Free PMC article. Review.
References
-
- Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
-
- Benard V, Bohl B P, Bokoch G M. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. - PubMed
-
- Blangy A, Vignal E, Schmidt S, Debant A, Gauthier-Rouviere C, Fort P. TrioGEF1 controls Rac- and Cdc42-dependent cell structures through the direct activation of rhoG. J Cell Sci. 2000;113:729–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
